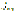2-Propenamide literature
Ru(ii)- And Ru(iv)-dmso complexes catalyze efficient and selective aqueous-phase nitrile hydration reactions under mild conditions
Dubey, Santosh Kumar,Kaur, Gurmeet,Rath, Nigam P.,Trivedi, Manoj
, p. 17339 - 17346 (2021/10/08)
New water-soluble ruthenium(ii)- and ruthenium(iv)-dmso complexes [RuCl2(dmso)2(NH3)(CH3CN)] (1), [RuCl2(dmso)3(CH3CN)] (2), and [RuCl2(dmso)3(NH3)]·PF6·Cl (3) have been synthesized and characterized using elemental analyses, IR, 1H and 31P NMR, and electronic absorption spectroscopy. The molecular structures of complexes 1-3 were determined crystallographically. The reactivity of complexes 1-3 has been tested for aqueous-phase nitrile hydration at 60 °C in air, and good efficiency and selectivity are shown for the corresponding amide derivatives. Best performance is achieved with complex 3. Amide conversions of 56-99% were obtained with a variety of aromatic, alkyl, and vinyl nitriles. The reaction tolerated hydroxyl, nitro, bromo, formyl, pyridyl, benzyl, alkyl, and olefinic functional groups. Amides were isolated by simple decantation from the aqueous-phase catalyst. A catalyst loading down to 0.0001 mol% was examined and turnover numbers as high as 990?000 were observed. The catalyst was stable for weeks in solution and could be reused more than seven times without significant loss in catalytic activity. The gram-scale reaction was also performed to produce the desired product in high yields. This journal is
Reactivity of secondary N-alkyl acrylamides in Morita–Baylis–Hillman reactions
Ahmar, Mohammed,Queneau, Yves,Verrier, Charlie,Yue, Xiaoyang
, p. 319 - 330 (2021/10/29)
The Morita–Baylis–Hillman (MBH) reaction of secondary N-alkyl acrylamides, discarded up to now from investigations of the scope of activated alkenes, was studied. Optimization of the reaction conditions revealed that a balance must be found between activation of the MBH coupling reaction and that of the undesired competitive aldehyde Cannizzaro reaction. Using 3-Hydroxyquinuclidine (3-HQD) in a 1:1 water-2-MeTHF mixture provides the appropriate conditions that were applicable to a wide range of diversely substituted secondary N-alkyl acrylamides and aromatic aldehydes, giving rise to novel amide-containing MBH adducts under mild and clean conditions.
INTEGRATED METHODS AND SYSTEMS FOR PRODUCING AMIDE AND NITRILE COMPOUNDS
-
Paragraph 00100, (2020/09/30)
Provided herein are integrated methods and systems for the production of acrylamide and acrylonitrile compounds and other compounds from at least beta-lactones and/or beta-hydroxy amides.
Simultaneous generation of acrylamide, β-carboline heterocyclic amines and advanced glycation ends products in an aqueous Maillard reaction model system
Chen, Jie,He, Zhiyong,Jiao, Ye,Li, Yong,Liu, Guoping,Qin, Fang,Quan, Wei,Wang, Zhaojun,Xue, Chaoyi,Zeng, Maomao
, (2020/07/06)
The simultaneous formation of acrylamide; β-carboline heterocyclic amines (HAs): harmane and norharmane; and advanced glycation end products (AGEs) (Nε-(carboxymethyl)lysine (CML) and Nε-(carboxyethyl)lysine (CEL)) was analyzed based on an aqueous model system. The model systems included lysine–glucose (Lys/Glu), asparagine–glucose (Asn/Glu), tryptophan–glucose (Trp/Glu), and a mixture of these amino acids (Mix/Glu). Only AGEs were generated when heated at 100 °C, Asn and Trp competed with Lys for glucose and methylglyoxal (MGO), and glyoxal (GO) decreased AGE content. The k value of CML, CEL, and acrylamide decreased when heated at 130 °C, whereas that of harmane increased in the Mix/Glu, owing to the competition between Lys and Asn for glucose, GO, and MGO. Harmane preferably formed via the Pictet–Spengler condensation between Trp and acetaldehyde, which further reduced acrylamide formation via the acrolein pathway.