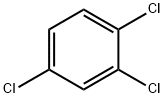
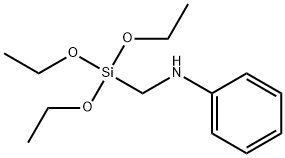
1,8-Diamino-3,6-dioxaoctane Application
Polyurethane industry, molecular sieves and catalyst carriers, pharmaceutical and biological products, surface treatment agents, lubricants and additives, battery technology
1,8-Diamino-3,6-dioxaoctane literature
A CHIRAL RESOLUTION METHOD MIMICKING MAGNETIC BENEFICIATION AND THE MAGNETIC NANO-INHIBITORS FOR SELECTIVE ENRICHMENT
-
, (2021/06/04)
A core-shell nanocomposite is formed by co-assembly of an amphiphilic polymer and hydrophobically modified magnetic nanoparticles, with its core being a hydrophobically modified magnetic nanomaterial and its shell being the amphiphilic polymer, wherein hydrophilic segments in the amphiphilic polymer are located at an outermost layer of the shell. The above composite can be used as additives in the crystallization of conglomerates and obtain optically pure crystals of both enantiomers in a single process. The key thereof is that the composite is used to enrich molecules with the same configuration while inhibit the crystallization of the other enantiomer in a supersaturated solution of conglomerates, such that a non-magnetic crystal and a magnetic crystal (which are enantiomers of each other) are generated in a unit operation. Optically pure crystals of both enantiomers with over 90 ee % can be obtained by one-time crystallization, and the total yield can be as high as 40%.
Method for preparing 1, 8 -diamino -3 and 6 -dioxaoctane through hydrogenation of triethylene glycol
-
Paragraph 0024-0039, (2021/09/08)
The invention belongs to the field of fine chemical engineering, and provides a method for preparing 1, 8 -diamino -3 and 6 -dioxaoctane through hydrogen ammonification of triethylene glycol. The liquid ammonia and the secondary amine inhibitor are mixed uniformly in the storage tank according to a certain proportion, Ni / Al is injected into the plunger pump. 2 O3 A fixed bed reactor of the catalyst is subjected to ammoniation reaction under a certain process condition. Finally, the product is introduced into the gas-liquid separator to separate 1, 8 - diamino -3, 6 - dioxolane. The invention innovatively utilizes the secondary amine inhibitor and to pre-activate the catalyst to improve the reaction performance and lower the reaction pressure.
Mild deprotection of the: N-tert -butyloxycarbonyl (N -Boc) group using oxalyl chloride
Awuah, Samuel G.,George, Nathaniel,Ofori, Samuel,Parkin, Sean
, p. 24017 - 24026 (2020/07/23)
We report a mild method for the selective deprotection of the N-Boc group from a structurally diverse set of compounds, encompassing aliphatic, aromatic, and heterocyclic substrates by using oxalyl chloride in methanol. The reactions take place under room temperature conditions for 1-4 h with yields up to 90percent. This mild procedure was applied to a hybrid, medicinally active compound FC1, which is a novel dual inhibitor of IDO1 and DNA Pol gamma. A broader mechanism involving the electrophilic character of oxalyl chloride is postulated for this deprotection strategy. This journal is
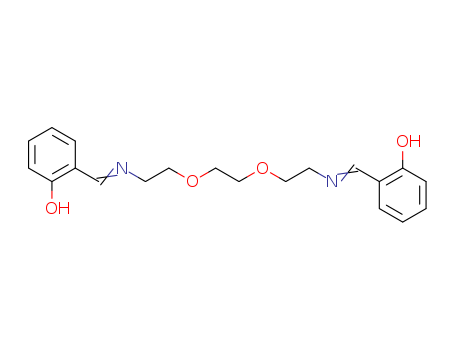
A novel graphite-like stacking structure in a discrete molecule and its molecular recognition behavior
Akine, Shigehisa,Onuma, Takahiro,Nabeshima, Tatsuya
supporting information, p. 9369 - 9372 (2018/06/18)
A graphite-like stacking structure was nicely reproduced in a discrete molecule that was prepared by 2+2 macrocyclic Schiff base formation. In the crystal structure, two hexabenzocoronene planes are closely stacked with displacement, yielding the intramolecular stacking structure similar to an AB- or ABC-stacking pattern in natural graphite. This molecule showed a recognition ability toward electron-deficient aromatic molecules in solution.