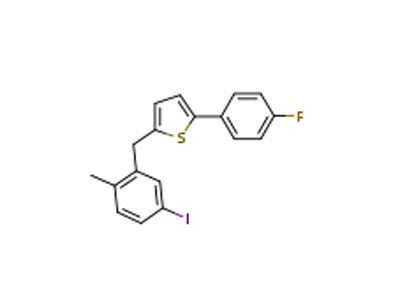
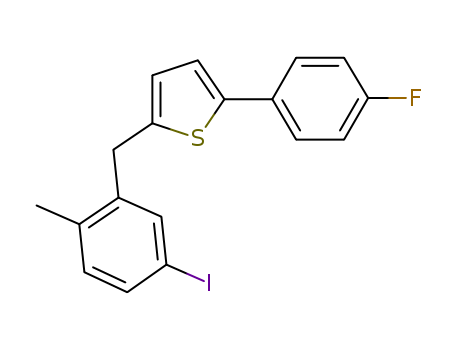
2-(4-Fluorophenyl)-5-[(5-iodo-2-methylphenyl)methyl]thiophene literature
Preparation method of carbagliflozin intermediate and application of intermediate in preparation of glipizide
-
Paragraph 0043; 0067; 0070-0073; 0076-0079; 0082; ..., (2021/11/26)
The invention provides a preparation method of a carbagliflozin intermediate and an application thereof in preparation of glipizide. To the preparation method, 5 - iodine -2 - methylbenzoic acid and a chlorination reagent are subjected to a Fourier acylation reaction, 5 - iodine -2 - methylbenzoyl chloride is obtained. The 5 -iod -2 -methylbenzoyl chloride and 2 - (4 - fluorophenyl) thiophene were subjected to a Fries alkylation reaction to give (5 -iod -2 -methylphenyl) (5 - (4 - fluorophenyl) thiophene -2 -yl) methyl ketone. (5 -iod -2 -methylphenyl) (5 - (4 - fluorophenyl) thiophene -2 -yl) methyl ketone was subjected to a carbonyl reduction reaction to give a crude product of. The intermediate crude product is subjected to purification treatment to obtain the glipizide intermediate. The preparation method has the advantages of mild reaction conditions, simple operation, environmental protection, safety, suitability for industrial mass production, and high product yield and purity.
METHOD FOR MANUFACTURING DIARYLMETHANE COMPOUND
-
Paragraph 0192-0215, (2021/07/02)
An object is to provide a method for producing a compound which is useful as a synthetic intermediate for an active pharmaceutical ingredient of an antidiabetic drug or the like in an industrially inexpensive and efficient manner, and the present invention can achieve the object by reducing a compound (2) represented by the following formula (2): wherein R1, Ar, n and X are as mentioned herein in the presence of a titanium compound by using a reducing agent to produce a compound (1) represented by the following formula (1): wherein R1, Ar and n are the same as defined above.
SYNTHESIS, CRYSTAL STRUCTURE, ANTI-LUNG CANCER ACTIVITY OF 2-(4-FLUOROPHENYL)-5- (5-IODO-2-METHYLBENZYL)THIOPHENE
Dong, Y. L.,Liu, J. P.,Qiu, F.,Wang, C. M.,Zhang, Z. F.,Zhou, L. P.
, p. 1111 - 1116 (2020/09/09)
Abstract: New heterocycle compound 2-(4-fluorophenyl)-5-(5-iodo-2-methylbenzyl)thiophene (1), designed using5-iodo-2-methylbenzoic acid (2) as the starting material is successfully obtained via the multiple synthesis route and finally characterized by IR, 1H NMR, and single crystal X-ray crystallography. In addition, the in vitro anticancer activity of compound 1 on three human lung cancer cells (H20, H2227, and H69) is further determined, which suggests that compound 1 may be a potential anticancer agent.
Synthesis method of canagliflozin intermediate
-
, (2018/03/26)
The invention relates to a synthesis method of a canagliflozin intermediate. Concretely, succinyl oxide and fluorobenzene are used as starting raw materials to be prepared into the canagliflozin crucial intermediate of 2-(4-fluorophenyl)-5-[(5-halogen-2-methyl phenyl)methyl]thiophene through the steps of Friedel-Crafts acylation ring opening, thiophene ring preparation, Friedel-Crafts acylation coupling, reduction and the like. The cheap succinyl oxide is used for preparing the thiophene ring, so that the Suzuki coupling reaction and Grignard reaction are avoided; the use of heavy metal reagents of palladium and the like is avoided; the production process is simplified; the product yield and the quality are improved; the environment pollution is reduced; the production cost is reduced.