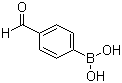
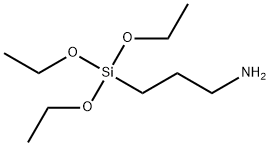
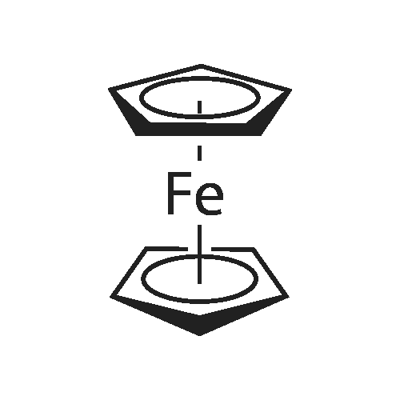
4-Formylphenylboronic acid literature
Space-group revision for 4-formylphenylboronic acid
Fronczek, Frank R.,St Luce, Nadia N.,Strongin, Robert M.
, (2001)
-
Argentivorous Molecules with Chromophores in Side Arms: Silver Ion-Induced Turn on and Turn off of Fluorescence
Ju, Huiyeong,Taniguchi, Aya,Kikukawa, Kaoru,Horita, Hiroki,Ikeda, Mari,Kuwahara, Shunsuke,Habata, Yoichi
supporting information, p. 9141 - 9147 (2021/06/28)
The synthesis of argentivorous molecules (L1 and L2) having two chromophores (4-(anthracen-9-yl)benzyl or 4-(pyren-1-yl)benzyl groups) and two benzyl groups and the fluorescence properties of their silver complexes in a solution and the solid state are reported. A crystallographic approach for the Ag+ complexes with L1 and L2 revealed that the observed fluorescence changes stem from the excimer formation and extinction of fluorescent. Furthermore, binding stabilities of L1 and L2 toward Ag+ ions were estimated by the Ag+-induced UV-vis and PL spectral changes.
Hydroxyl radical-mediated oxidative cleavage of CC bonds and further esterification reaction by heterogeneous semiconductor photocatalysis
Hong, Mei,Jia, Rui,Miao, Hongyan,Ni, Bangqing,Niu, Tengfei,Wang, Hui
, p. 6591 - 6597 (2021/09/10)
A hydroxyl radical-mediated aerobic cleavage of alkenes and further sequence esterification reaction for the preparation of carbonyl compounds have been developed by using tubular carbon nitride (TCN) as a general heterogeneous photocatalyst under an oxygen atmosphere with visible light irradiation. This protocol has an excellent substrate scope and gives the desired aldehydes, ketones and esters in moderate to high yields. Importantly, this metal-free procedure employed photogenerated hydroxyl radicals in situ as green oxidation active species, avoiding the present additional initiators. The reaction could be carried out under solar light irradiation and was applicable to large-scale reactions. Furthermore, the recyclable TCN catalyst could be used several times without a significant loss of activities.
Enantioselective Conjugate Addition of Aryl Halides and Triflates to Electron-Deficient Olefins via Nickel- And Rhodium-Catalyzed Sequential Relay Reactions
Fan, Chenrui,Wu, Qixu,Zhu, Chengfeng,Wu, Xiang,Li, Yougui,Luo, Yunfei,He, Jian-Bo
supporting information, p. 8888 - 8892 (2019/10/14)
Asymmetric conjugate addition of aryl halides or aryl triflates to electron-deficient olefins was realized by sequential Miyaura borylation and Hayashi-Miyaura conjugate addition in one pot. A nickel-catalyzed borylation of aryl halides or triflates and a rhodium-chiral diene complex catalyzed enantioselective conjugate addition was executed as a pair of relay reactions as a more efficient and greener protocol.
Method for synthesizing aromatic aldehyde through iron catalyzed oxidation allyl aromatic compound
-
Paragraph 0039-0041; 0149, (2019/06/27)
The invention discloses a method for synthesizing aromatic aldehyde through an iron catalyzed oxidation allyl aromatic compound. According to the specific method, under the promotion effect of hydrogen silane, with air or oxygen as the oxidant, the aromatic aldehyde compound is synthesized through the iron catalyzed oxidation allyl aromatic compound, the reaction temperature is 20-150 DEG C, and the time is 0.25-60 h. The method has the advantages that a catalyst source is wide, the price is low and the environment is protected; an oxidant source is wide, the price is low and no waste is generated; the reaction conditions are mild, selectivity is high and the yield is high; a substrate source is wide and stable; a substrate functional group is high in compatibility and a substrate is widein application range; complicated small molecules are compatible and can be well converted into aldehyde. The target product separation yield can reach up to 96% under the optimized reaction conditions.