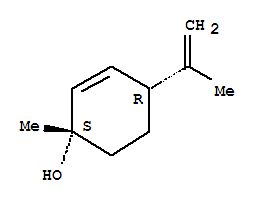
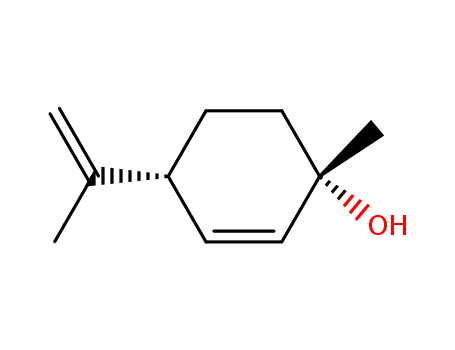
(+)-(1S,4R)-P-MENTHA-2,8-DIEN-1-OL literature
Highly efficient catalysts in directed oxygen-transfer processes: Synthesis, structures of novel manganese-containing heteropolyanions, and applications in regioselective epoxidation of dienes with hydrogen peroxide
B?sing, Michael,N?h, Andreas,Loose, Ina,Krebs, Bernt
, p. 7252 - 7259 (1998)
A series of novel manganese(II)-substituted polyoxometalates, [(M(II)(H2O)3)2(WO2)2(BiW9O33)2] 10-(1), [(Mn(II)(H2O))3(SbW9O33)2]12- (2), and [(Mn(II)(H2O)3)2(Mn(II)(H2O)2)2(TeW9O33)2]8- (3), were synthesized and characterized by X-ray structure analyses. The use of these oxidatively and solvolytically stable heteropolyanions as homogeneous catalysts for the epoxidation of dienes was investigated by gas chromatography/mass spectrometry, IR spectroscopy, UV-visible studies, and cyclic voltammetric measurements. The catalytic performance is exemplified by the model substrate (R)-(+)-limonene, at ambient temperatures in a biphasic system, with excellent regioselectivities, >99%, and very high turnovers even with only a small molar excess of hydrogen peroxide.
Stereoselective Synthesis of Nonpsychotic Natural Cannabidiol and Its Unnatural/Terpenyl/Tail-Modified Analogues
Anand, Radhika,Cham, Pankaj Singh,Gannedi, Veeranjaneyulu,Sharma, Sumit,Kumar, Mukesh,Singh, Rohit,Vishwakarma, Ram A.,Singh, Parvinder Pal
, p. 4489 - 4498 (2022/04/07)
Here, we report a three-step concise and stereoselective synthesis route to one of the most important phytocannabinoids, namely, (-)-cannabidiol (-CBD), from inexpensive and readily available starting material R-(+)-limonene. The synthesis involved the diastereoselective bifunctionalization of limonene, followed by effective elimination leading to the generation of key chiral p-mentha-2,8-dien-1-ol. The chiral p-mentha-2,8-dien-1-ol on coupling with olivetol under silver catalysis provided regiospecific (-)-CBD, contrary to reported ones which gave a mixture. The newly developed approach was further extended to its structural analogues cannabidiorcin and other tail/terpenyl-modified analogues. Moreover, its opposite isomer (+)-cannabidiol was also successfully synthesized from S-(-)-limonene.
Preparation method of (1S, 4R)-1-methyl-4-(1-methylvinyl)-2-cyclohexene-1-ol
-
, (2021/04/17)
The invention relates to a preparation method of (1S, 4R)-1-methyl-4-(1-methylvinyl)-2-cyclohexene-1-ol, and belongs to the technical field of organic solvents. The preparation method comprises the following steps: reacting limonene with a halogenating reagent to obtain an intermediate shown in a formula (1), then reacting the intermediate shown in the formula (1) with a tertiary amine compound to obtain an intermediate shown in a formula (2), and finally carrying out a pyrolysis elimination reaction on the intermediate shown in the formula (2) to obtain (1S, 4R)-1-methyl-4-(1-methylvinyl)-2-cyclohexene-1-ol. The preparation method has the advantages of mild conditions, no need of an oxidation process of hydrogen peroxide with high preparation condition requirements and high safety risk, no need of an expensive catalyst, high yield, high product purity, simple steps, and suitableness for industrial production.
CANNABINOID DERIVATIVES, PRECURSORS AND USES
-
, (2021/03/19)
The present disclosure relates to new cannabinoid derivatives and precursors and processes for their preparation. The disclosure also relates to pharmaceutical and analytical uses of the new cannabinoid derivatives.
Preparation method of (1S, 4R)-1-methyl-4-(1-methyl vinyl)-2-cyclohexene-1-alcohol
-
, (2021/01/15)
The invention discloses a preparation method of (1S, 4R)-1-methyl-4-(1-methyl vinyl)-2-cyclohexene-1-alcohol, which comprises the following steps: by using D-limonene as a raw material, carrying out double bond addition and hydroxyl protection to obtain an intermediate compound; and carrying out elimination reaction and continuing deprotection to finally prepare the product. The method has the advantages of two-step continuous reaction, short process route and few byproducts, the obtained product has high chiral selectivity, and the yield is up to 70% or above and is greatly improved comparedwith the existing process.