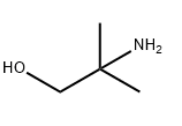
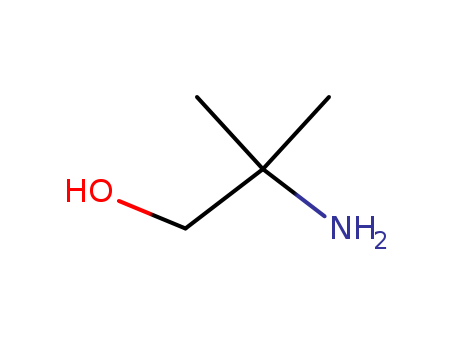
2-Amino-2-methyl-1-propanol literature
A simple total synthesis of naturally occurring hydroxy-amino acids by enzymatic kinetic resolution
Lu,Miet,Kunesch,Poisson
, p. 893 - 902 (1993)
Both optically pure enantiomers of GABOB and isoserine were obtained by enzymatic kinetic resolution of acetylated precursors in three or four steps. The key intermediates were cyanohydrins available from simple aldehydes. This procedure can be applied to other unusual hydroxy amino acids widely distributed in biologically important peptides.
Cyclization of vinyl ethers derived from amino alcohols
Kukharev,Stankevich,Klimenko,Kukhareva
, p. 966 - 969 (2007)
Cyclization of vinyl ethers derived from linear and cyclic α- and β-amino alcohols, catalyzed by mercury(II) acetate gave 2-methyloxazolidines and 2-methylperhydro-1,3-oxazines in 37-94% yield.
Synthesis and characterization of lanthanide complexes prepared with 2-((E)-(1-hydroxy-2-methylpropan-2-ylimino)methyl)-6-methoxyphenol
Abrahams, Abubak'r,Madanhire, Tatenda,Hosten, Eric,Betz, Richard
, p. 1994 - 2014 (2017)
Rare-earth complexes of the general formula [Ln(H2L1)2(NO3)3] [Ln?=?Gd (1), Ho (2) or Nd (3)] were prepared from an o-vanillin derived Schiff base ligand, 2-((E)-(1-hydroxy-2-methylpropan-2-ylimino)methyl)-6-methoxyphenol (H2L1). The single-crystal X-ray diffraction studies and SHAPE analyses of the Gd(III) and Ho(III) complexes show that the complexes are ten-coordinate and exhibit distorted tetradecahedron geometries. The phenolate oxygen-bridged dinuclear complex, [Ce2(H2L1)(ovan)3(NO3)3] (4, ovan?=?monodeprotonated o-vanillin), was obtained from the reaction of Ce(NO3)3?6H2O with H2L1. X-ray analysis revealed that hydrolysis of H2L1 occurred to yield o-vanillin, which bridged two cerium atoms with the Ce?Ce distance equal to 3.8232(6) ?. The Ce(III) ions are both ten-coordinate, but have different coordination environments, showing tetradecahedron and staggered dodecahedron geometries, respectively. With proton migration occurring from the phenol group to the imine function, complexation of the lanthanides to the ligand gives the Schiff base a zwitterionic phenoxo-iminium form.
Preparation method of 2-amino-2-methyl-1-propanol
-
Paragraph 0056-0059, (2021/03/11)
The invention discloses a preparation method of 2-amino-2-methyl-1-propanol, which comprises the following preparation steps: adding 2, 2-dimethyl aziridine into a slightly acidic water system, and reacting at the temperature of below 50 DEG C to obtain a reactant; and neutralizing, roughly distilling and rectifying the reactant to obtain the 2-amino -2-methyl-1-propanol. The method has the advantages of mild reaction conditions, simplicity in operation, easiness in mastering, easiness in treatment of the reaction product, high product purity (the purity is 99.0% or above), high yield (the total yield is 90% or above).
Copper(I) Phosphinooxazoline Complexes: Impact of the Ligand Substitution and Steric Demand on the Electrochemical and Photophysical Properties
Frey, Wolfgang,Giereth, Robin,Karnahl, Michael,Klo?, Marvin,Mengele, Alexander K.,Steffen, Andreas,Tschierlei, Stefanie
, p. 2675 - 2684 (2020/03/04)
A series of seven homoleptic CuI complexes based on hetero-bidentate P^N ligands was synthesized and comprehensively characterized. In order to study structure–property relationships, the type, size, number and configuration of substituents at the phosphinooxazoline (phox) ligands were systematically varied. To this end, a combination of X-ray diffraction, NMR spectroscopy, steady-state absorption and emission spectroscopy, time-resolved emission spectroscopy, quenching experiments and cyclic voltammetry was used to assess the photophysical and electrochemical properties. Furthermore, time-dependent density functional theory calculations were applied to also analyze the excited state structures and characteristics. Surprisingly, a strong dependency on the chirality of the respective P^N ligand was found, whereas the specific kind and size of the different substituents has only a minor impact on the properties in solution. Most importantly, all complexes except C3 are photostable in solution and show fully reversible redox processes. Sacrificial reductants were applied to demonstrate a successful electron transfer upon light irradiation. These properties render this class of photosensitizers as potential candidates for solar energy conversion issues.
PROCESS FOR PRODUCING SUBSTITUTED AMINO ALCOHOLS
-
Page/Page column 33-36, (2020/06/01)
The present invention relates to a process for producing a compound of the formula (I) comprising at least the process step: a) reacting a compound of the formula (II) with hydrogen and water in the presence of at least one homogeneous transition metal catalyst TMC 1.
Palladium-Catalyzed Oxidation of β-C(sp3)-H Bonds of Primary Alkylamines through a Rare Four-Membered Palladacycle Intermediate
Bunescu, Ala,Ernst, Martin,Hartwig, John F.,Qiu, Yehao,Su, Bo,Zuend, Stephan J.
supporting information, p. 7912 - 7919 (2020/05/22)
Site-selective functionalizations of C-H bonds are often achieved with a directing group that leads to five- or six-membered metallacyclic intermediates. Analogous reactions that occur through four-membered metallacycles are rare. We report a challenging palladium-catalyzed oxidation of primary C-H bonds β to nitrogen in an imine of an aliphatic amine, a process that occurs through a four-membered palladacyclc intermediate. The success of the reaction relies on the identification, by H/D exchange, of a simple directing group (salicylaldehyde) capable of inducing the formation of this small ring. To gain insight into the steps of the catalytic cycle of this unusual oxidation reaction, a series of mechanistic experiments and density functional theory (DFT) calculations were conducted. The experimental studies showed that cleavage of the C-H bond is rate-limiting and formation of the strained four-membered palladacycle is thermodynamically uphill. DFT calculations corroborated these conclusions and suggested that the presence of an intramolecular hydrogen bond between the oxygen of the directing group and hydroxyl group of the ligating acetic acid is crucial for stabilization of the palladacyclic intermediate.