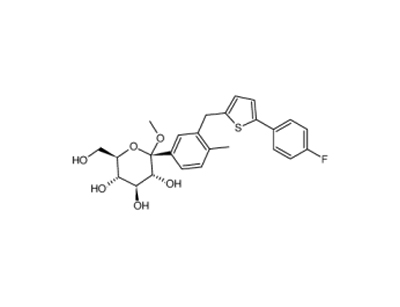
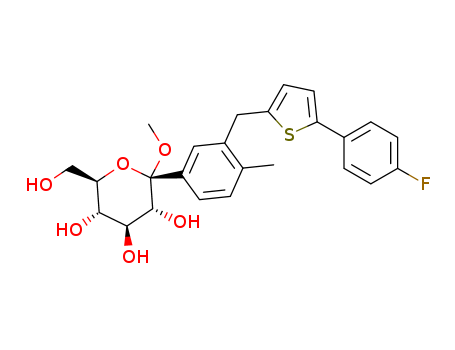
(2S,3R,4S,5S,6R)-2-(3-((5-(4-fluorophenyl)thiophen-2-yl)Methyl)-4-Methylphenyl)-tetrahydro-6-(hydroxyMethyl)-2-Methoxy-2H-pyran-3,4,5-triol literature
Telescoped lithiation, C-arylation and methoxylation in flow-batch hybrid toward the synthesis of canagliflozin
Hone, Christopher A.,Oliver Kappe, C.,Polterauer, Dominik,Williams, Jason D.
supporting information, (2021/09/22)
We report a highly efficient three-step flow-batch hybrid procedure for the synthesis of a key canagliflozin intermediate. The telescoped process provides exquisite control over an exothermic and mixing sensitive lithiation and subsequent C-arylation within a microstructured flow reactor. Methoxylation reagents are then added in flow, before reaching completion in a batch vessel. The flow process afforded the target intermediate in 76% yield, with a throughput of 26.8 g/h.
Preparation method and application of canagliflozin alpha isomer
-
Paragraph 0019; 0027-0030, (2021/01/25)
The invention discloses a preparation method and application of a canagliflozin alpha isomer, the preparation method comprises the following steps: in the presence of Lewis acid, a compound of formulaIV and a reducing agent are subjected to a reduction reaction in an organic solvent to obtain the canagliflozin alpha isomer, wherein the lewis acid is a mixture of trifluoroacetic acid and trimethylsilyl trifluoromethanesulfonate; the reducing agent is 1, 1, 3, 3-tetramethyldisiloxane. The preparation method is simple, the high-purity canagliflozin related substance reference substance can be rapidly obtained, the yield of the product canagliflozin alpha isomer is 37.5%, the purity is as high as 97.61%, the purity requirement of a standard substance is completely met, and the canagliflozin alpha isomer can be used as the standard substance and can be applied to quality control and research of canagliflozin bulk drugs. Therefore, the quality control level of canagliflozin is improved, andthe clinical medicinal safety is ensured.
Method for preparing intermediates of gliflozin hypoglycemic drugs
-
Paragraph 0043; 0046-0047; 0050, (2020/05/02)
The invention belongs to the technical field of medicine synthesis, and relates to a novel method for preparing key intermediates of gliflozin hypoglycemic drugs, in particular to a preparation methodof key intermediates (C-1, D-1 and E-1) of canagliflozin, dapagliflozin and empagliflozin. The method comprises the following steps: 1) in the presence of a cosolvent, carrying out halogen metal exchange on a raw material, namely aryl bromide 2 and an organic lithium reagent to obtain an aryl lithium reagent 3, and carrying out a nucleophilic addition reaction on the aryl lithium reagent 3 and TMS-protected glucolactone 4 to obtain a transition product 5; and 2) removing a TMS protecting group from the transition product 5, and converting hemiketal into ketal to obtain the key intermediate 1with a single configuration. According to the method, the key intermediates (C-1, D-1 and E-1) of canagliflozin, dapagliflozin and empagliflozin can be stereoselectively synthesized, reaction yield isrelatively high (more than 75%), and product purity is high (wherein HPLC purity is about 95%); so reduction preparation of a final product in the next step is facilitated.
Preparation method of high-purity canagliflozin intermediate
-
Paragraph 0045; 0047-0060; 0062-0073; 0076-0078; 0081, (2020/08/30)
The invention discloses a preparation method of a high-purity canagliflozin intermediate, which comprises the following steps: reacting a thiophene compound with an alkaline reagent in an inert environment at low temperature, and condensing the reaction product with 2, 3, 4, 6-tetra-O-(trimethylsilyl)-D-glucolactone; dropwise adding a strong acid aqueous solution into the obtained reaction solution, quenching after reaction, separating out an organic phase for concentrating, and performing crystallizing and drying to obtain an intermediate I; reacting the intermediate I with strong acid in methanol to obtain a high-purity canagliflozin intermediate II. By preparing the intermediate I with excellent crystallization performance, the purity of the canagliflozin intermediate II is improved. The operation is simple and convenient, the production process is stable, and industrial production is facilitated.