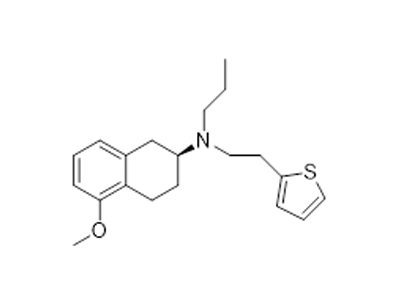
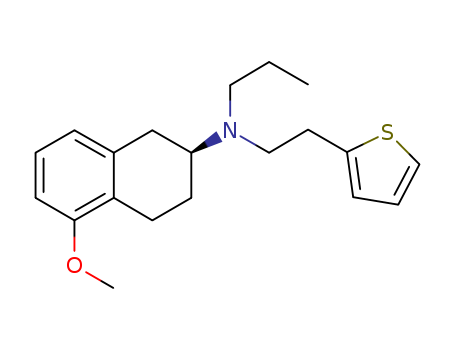
(S)-5-Methoxy-N-propyl-N-(2-(thiophen-2-yl)ethyl)-1,2,3,4-tetrahydronaphthalen-2-aMine literature
Preparation method of rotigotine
-
, (2021/08/11)
The invention relates to the technical field of medicine preparation, and discloses a preparation method of rotigotine, which comprises the following steps: by taking 5-methoxy-2-tetralone as an initial raw material, reacting with R-alpha-methylbenzylamine, performing debenzylation reduction and S-mandelic acid chiral resolution, then reacting with a propionyl chloride reagent to generate an amide compound, and then reducing by a sodium borohydride reagent to obtain the rotigotine; and finally, reacting with 2-(thiophene-2-yl) 2-nitric acid benzene sulfonic acid ethyl ester to obtain the rotigotine. The preparation process route is as follows: the rotigotine is mild in preparation condition, simple and convenient to operate, relatively high in yield of key intermediates, high in optical purity and easy for industrial large-scale production, and has a very good application prospect.
Preparation method for rotigotine
-
, (2019/04/17)
The invention discloses a preparation method for rotigotine. The preparation method includes the following steps: S1. performing an amination reduction reaction on a 5-methoxy-2-tetralone solution, tert-butanesulfinamide, a catalyst, and sodium borohydride to obtain a substance A; S2. performing an alkylation reaction on a solution of the substance A, bromopropane, and a basic catalyst to obtain asubstance B; S3. reacting the substance B with a hydrochloric acid methanol solution to obtain a substance C; S4. performing a reaction on the substance C, 2-(2-bromoethyl)thiophene, potassium carbonate, and N,N-dimethylformamide to obtain a substance D; and S5. reacting acetic acid with hydrogen bromide to obtain the rotigotine. The preparation method is simple in operation, is high in yield, ismild in reaction condition, is green and environmentally friendly, is high in purity of the prepared rotigotine, and is suitable for large-scale industrial production.
luo Ti gastrodia tuber of a kind of preparation method
-
, (2017/02/24)
The invention discloses a preparation method of rotigotine, and belongs to the technical field of medicine synthesis. According to the method, 5-methoxy-2-tetralone is used as a raw material for amination, asymmetric reduction, halogenation and methoxyl group removal four step reaction for synthesis of chiral rotigotine {(-)-(S)-2-(N-propyl-N-(2-(2-thiophene) ethyl] amino]-5-hydroxy-1, 2, 3, 4-tetralin}. According to the method, a simplex stereoscopic structural compound is synthesized by stereo selective chemical reaction, in the asymmetric reduction process, hantzsch ester 1, 4-dihydropyridine (HEH) is used as a reducing agent, and chiral phosphoric acid is used as a catalyst to synthesize an important intermediate (S)-2-(N-n-propyl) amido-5-methoxy tetralin (II) with a simplex stereoscopic structure, the use of a chiral reagent for splitting to obtain the simplex stereoscopic structural compound is avoided, the synthesis procedure is shortened, the yield is improveds, and the method is favorable for industrialized production.
S-5-SUBSTITUENT-N-2'-(THIOPHENE-2-YL)ETHYL-TETRALIN-2-AMINE OR CHIRAL ACID SALTS THEREOF AND USE FOR PREPARING ROTIGOTINE
-
Paragraph 0051, (2013/03/26)
The chiral compound S-5-substituted-N-2′-(thienyl-2-yl-)ethyl-tetralin-2-amine or its chiral acid salts and preparation method thereof are disclosed, and the method for preparing Rotigotine by using the chiral compound is also disclosed. Racemic 5-substituted-N-2′-(thien-2-yl-)ethyl-tetralin-2-amine (compound 1) is resolved by using a conventional chiral acid to obtain an optically pure chiral acid salt of S-5-substituted-N-2′-(thien-2-yl-)ethyl-tetralin-2-amine, which is then dissociated to obtain S-5-substituted-N-2′-(thien-2-yl-)ethyl-tetralin-2-amine (compound 2). The compound 2 or chiral acid salt thereof is alkylated and deprotected to produce rotigotine (compound 5).