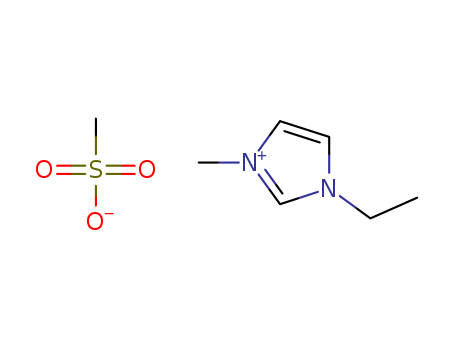
1-ETHYL-3-METHYLIMIDAZOLIUM METHANESULFONATE literature
Redox Reaction: A New Route for the Synthesis of Water-Miscible Imidazolium Ionic Liquids
Li, Wenxiu,Dai, Shangwu,Li, Dong,Zhang, Qinqin,Fan, Hongtao,Zhang, Tao,Zhang, Zhigang
, p. 1065 - 1072 (2017/02/23)
A novel chemical redox route was developed for the preparation of water-miscible imidazolium ionic liquids (ILs). In this method, the reaction between 1-alkyl-3-methylimidazolium bromides or 3-butyl-1-phenylimidazolium bromide and the appropriate acid reactant was promoted by the redox reaction between the bromide ion and aqueous hydrogen peroxide, with hex-1-ene as both solvent and bromine scavenger. The residual bromide ion and water contents of the prepared ILs were determined by ion chromatography and the Karl-Fischer test, respectively. This method not only produces water-miscible ILs in high purity and high yield, but also simplifies the reaction conditions in comparison with previous routes.
Bronsted acids in ionic liquids: How acidity depends on the liquid structure
McCune, Jade A.,He, Peizhao,Petkovic, Marina,Coleman, Fergal,Estager, Julien,Holbrey, John D.,Seddon, Kenneth R.,Swadba-Kwasny, Malgorzata
, p. 23233 - 23243 (2015/01/08)
Gutmann Acceptor Number (AN) values have been determined for Bronsted acid-ionic liquid mixtures, over a wide compositional range. Four systems of general formula [C2mim][A]-HA (A- = bistriflamide, [NTf2]-; triflate, [OTf]-; mesylate, [OMs]-; or acetate, [OAc]-, [C2mim]+ = 1-ethyl-3-methylimidazolium cation) were studied. A library of Bronsted acidic systems of varying acidity was constructed and the AN parameter was found to be a convenient approach for quantifying their acidity. HOAc, HOMs and HOTf, when dissolved in ionic liquids, were found to associate with the respective anions to form hydrogen-bonded anionic clusters, [A(HA)x]-. In contrast, HNTf2 was solubilised as a discrete, undissociated molecule. AN values were sensitive to the presence of anionic clusters; acidity could be buffered to a particular AN by binding the solubilised acid in the anionic cluster form. Overall, a simple way to manipulate and quantify the Bronsted acidity of acid-ionic liquid mixtures was demonstrated, and measured AN values were related to liquid speciation. This journal is
IONIC LIQUID CONTAINING ALLYLSULFONATE ANION
-
Page/Page column 19, (2012/06/30)
PROBLEM: Providing a novel ionic liquid, which is low-cost, environment-friendly, and has low viscosity and melting point. MEANS FOR SOLVING THE PROBLEM: The present invention is the invention of the ionic liquid represented by the general formula [1]: {wherein, R1 to R3 and n pieces of R4 each independently represent hydrogen atom or alkyl group having 1 to 4 carbon atoms, R5 to R7 each independently represent alkyl group, aralkyl group, or aryl group, R8 represents alkyl group, aralkyl group, aryl group, or the one represented by the general formula [2]: (wherein T represents alkylene chain having 1 to 8 carbon atoms, n represents 1 or 2, and R1 to R7 are the same as the above-described), X represents nitrogen atom or phosphorus atom, n represents 1 or 2. When n is 1, R3 and R4 are bound and may form cyclohexene ring together with the adjacent carbon atoms. In addition, when X is nitrogen atom, R5 to R7 or R5 to R6 may form hetero ring with nitrogen atom binding thereto}.
Liquid-liquid interfacial tension of equilibrated mixtures of ionic liquids and hydrocarbons
Rodriguez, Hector,Arce, Alberto,Soto, Ana
, p. 1519 - 1524 (2012/11/07)
Ionic liquids are possible alternative solvents for the separation of aromatic and aliphatic hydrocarbons by liquid-liquid extraction. Interfacial tension is an important property to consider in the design of liquid-liquid extraction processes. In this work, the liquid-liquid interfacial tension and the mutual solubility at 25 °C have been measured for a series of biphasic, equilibrated mixtures of an ionic liquid and a hydrocarbon. In particular, the ionic liquids 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl) imide (with the alkyl substituent being ethyl, hexyl or decyl), 1-ethyl-3- methylimidazolium ethylsulfate, and 1-ethyl-3-methylimidazolium methanesulfonate have been selected, as well as the hydrocarbons benzene, hexane, ethylbenzene, and octane. The selected sets of ionic liquids and hydrocarbons allow the analysis of the influence of a series of effects on the interfacial tension. For example, the interfacial tension decreases with an increase in the length of the alkyl substituent chain of the cation or with an increase of the degree of charge delocalisation in the anion of the ionic liquid. Also, the interfacial tension with the aromatic hydrocarbons is markedly lower than that with the aliphatic hydrocarbons. A smaller effect is caused by variation of the size of the hydrocarbon. Some of the observed trends can be explained from the mutual solubility of the hydrocarbon and the ionic liquid. Science China Press and Springer-Verlag Berlin Heidelberg 2012.