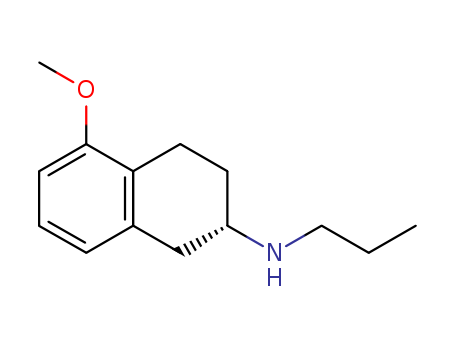
(S)-1,2,3,4-tetrahydro-5-methoxy-N-propyl-2-Naphthalenamine(Rotigotine) literature
New catalytic route for the synthesis of an optically active tetralone-derived amine for rotigotine
Cobley, Christopher J.,Evans, George,Fanjul, Tamara,Simmonds, Shaun,Woods, Amy
, p. 986 - 989 (2016)
Rotigotine is a launched drug for the treatment of Parkinson's disease and restless legs syndrome. The key steps of an alternative route for the synthesis of rotigotine have been demonstrated. Formation of a prochiral enamide, asymmetric hydrogenation of the enamide with high enantioselectivity, and reduction of the resulting amide to an amine have been proved to work successfully. The best conditions screened to date for the asymmetric hydrogenation of enamide 9 to amide 10 were with [(RuCl((R)-T-BINAP))2(μ-Cl)3][NH2Me2] at 25 bar H2 and 30 °C (500:1 S/C ratio, 99% conversion, 91% ee S). Reduction of amide 10 to amine 5 was best achieved with Red-Al giving 95% conversion.
Synthesis and pharmacology of the enantiomers of the potential atypical antipsychotic agents 5-OMe-BPAT and 5-OMe-(2,6-di-OMe)-BPAT
Homan, Evert J.,Copinga, Swier,Unelius, Lena,Jackson, David M.,Wikstroem, Hkan V.,Grol, Cor J.
, p. 1263 - 1271 (1999)
The optically pure enantiomers of the potential atypical antipsychotic agents 5-methoxy-2-[N-(2-benzamidoethyl)-N-n-propylamino]tetralin (5-OMe-BPAT, 5) and 5-methoxy-2-{N-[2-(2,6-dimethoxy)benzamidoethyl]-N-n-propylamino}tetralin [5-OMe-(2,6-di-OMe)-BPAT
Synthesis of Pharmaceutically Relevant 2-Aminotetralin and 3-Aminochroman Derivatives via Enzymatic Reductive Amination
Citoler, Joan,Harawa, Vanessa,Marshall, James R.,Bevinakatti, Han,Finnigan, James D.,Charnock, Simon J.,Turner, Nicholas J.
, p. 24456 - 24460 (2021/10/19)
2-Aminotetralin and 3-aminochroman derivatives are key structural motifs present in a wide range of pharmaceutically important molecules. Herein, we report an effective biocatalytic approach towards these molecules through the enantioselective reductive coupling of 2-tetralones and 3-chromanones with a diverse range of primary amine partners. Metagenomic imine reductases (IREDs) were employed as the biocatalysts, obtaining high yields and enantiocomplementary selectivity for >15 examples at preparative scale, including the precursors to Ebalzotan, Robalzotan, Alnespirone and 5-OH-DPAT. We also present a convergent chemo-enzymatic total synthesis of the Parkinson's disease therapy Rotigotine in 63 % overall yield and 92 % ee.
Preparation method of rotigotine
-
Paragraph 0064-0066, (2021/08/11)
The invention relates to the technical field of medicine preparation, and discloses a preparation method of rotigotine, which comprises the following steps: by taking 5-methoxy-2-tetralone as an initial raw material, reacting with R-alpha-methylbenzylamine, performing debenzylation reduction and S-mandelic acid chiral resolution, then reacting with a propionyl chloride reagent to generate an amide compound, and then reducing by a sodium borohydride reagent to obtain the rotigotine; and finally, reacting with 2-(thiophene-2-yl) 2-nitric acid benzene sulfonic acid ethyl ester to obtain the rotigotine. The preparation process route is as follows: the rotigotine is mild in preparation condition, simple and convenient to operate, relatively high in yield of key intermediates, high in optical purity and easy for industrial large-scale production, and has a very good application prospect.
Preparation method for rotigotine
-
, (2019/04/17)
The invention discloses a preparation method for rotigotine. The preparation method includes the following steps: S1. performing an amination reduction reaction on a 5-methoxy-2-tetralone solution, tert-butanesulfinamide, a catalyst, and sodium borohydride to obtain a substance A; S2. performing an alkylation reaction on a solution of the substance A, bromopropane, and a basic catalyst to obtain asubstance B; S3. reacting the substance B with a hydrochloric acid methanol solution to obtain a substance C; S4. performing a reaction on the substance C, 2-(2-bromoethyl)thiophene, potassium carbonate, and N,N-dimethylformamide to obtain a substance D; and S5. reacting acetic acid with hydrogen bromide to obtain the rotigotine. The preparation method is simple in operation, is high in yield, ismild in reaction condition, is green and environmentally friendly, is high in purity of the prepared rotigotine, and is suitable for large-scale industrial production.
Enantioselective Synthesis of β-Aminotetralins via Chiral Phosphoric Acid-catalyzed Reductive Amination of β-Tetralones
Park, Do Young,Kim, Kyung-Hee,Cheon, Cheol-Hong
, p. 462 - 467 (2017/12/07)
A new protocol for the synthesis of chiral β-aminotetralins has been developed via chiral phosphoric acid-catalyzed asymmetric reductive amination of β-tetralones using a Hantzsch ester as an organic hydride donor. Various chiral β-aminotetralins were obtained in good yields with good to high enantioselectivities. Furthermore, the utility of our new protocol was successfully demonstrated in the enantioselective synthesis of rotigotine. (Figure presented.).