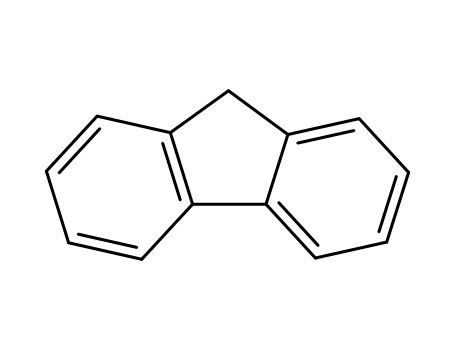
Fluorene literature
Synthesis and thermal rearrangement of pentacyclo[6.5.0.04,12.05,10.09,13]trideca-2,6-diene
Marchand,Rajapaksa,Vidyasagar,Eckrich,Kumar
, p. 11937 - 11944 (1995)
Pentacyclo[6.5.0.04,12.05,10.09,13]trideca-2,6-diene (16) has been synthesized in five steps from 1-hydroxyhexacyclo[6.5.0.02,6.03,11.05,10.09,12]trideca n-7-one (10). Compound 16 undergoes thermal rearrangement to pentacyclo[7.4.0.02,6.03,11.05,10]trideca-7,12-diene (i.e. '[2.2.1]triblattadiene', 19). The intermediacy of cis,cisoid,cis-tricyclo[7.4.0.02,7[trideca-3,5,10,12-tetraene (18) in the thermal rearrangement of 16 was inferred via analysis of the 1H NMR spectrum of partially rearranged 16 and subsequently was further established via the results of a trapping experiment (i.e., fluorene was produced when thermal rearrangement of 16 was performed in the presence of 10% Pd/C).
Carbanion Intermediates in the Photodecarboxylation of Benzannelated Acetic Acids in Aqueous Solution
McAuley, Iain,Krogh, Erik,Wan, Peter
, p. 600 - 602 (1988)
-
Hydrogenation of 9-pyridylmethylene- and 9-benzylidene(aza)fluorenes in the presence of rhenium heptasulfide
Kolyadina,Soldatenkov,Ryashentseva,Prostakov
, p. 171 - 173 (1996)
Hydrogenation of 9-pyridylmethylene(aza)fluorenes and 9-benzylidene-4-azafluorene at 250°C and pH2 = 130 atm in the presence of Re2S7 as a catalyst occurs preferably at the exocyclic double bond of the fulvene fragment to yield pyridyl-9-(aza)fluorenylmethanes.
Laser-induced carbene-carbene rearrangement in solution: The diphenylcarbene-fluorene rearrangement
Regimbald-Krnel, Michele J.,Wentrup, Curt
, p. 8789 - 8795 (2013)
Diphenylcarbene (DPC) generated by high-intensity laser photolysis of diphenyldiazomethane rearranges to fluorene (FL) by two distinct mechanisms as revealed by methyl-group labeling. Thus, excimer laser irradiation of p,p′-dimethyldiphenyldiazomethane generates 3,6-dimethylfluorene (3,6-DMF) and 2,7-dimethylfluorene (2,7-DMF), which were identified by fluorescence measurements as well as GC-MS and comparison with authentic materials. 3,6-DMF corresponds to direct bond formation between ortho positions in DPC, referred to as ortho,ortho′ coupling. 2,7-DMF corresponds to a carbene-carbene rearrangement, whereby DPC undergoes ring expansion to phenylcycloheptatetraene (PhCHT) followed by ring contraction to o-biphenylylcarbene (o-BPC), which then cyclizes to FL. The carbene-carbene rearrangement dominates over the ortho,ortho′ coupling under all conditions employed. The ortho,ortho′ coupling must take place in a higher excited state (most likely S2 or T1) of DPC, because it is not observed at all under thermolysis conditions, where only S1 and T0 are populated. The carbene-carbene rearrangement may take place either in a hot S1 state or more likely in a higher excited state (S2 or T1).
Surface confined ketyl radicals via samarium(II)-grafted mesoporous silicas [3]
Nagl, Iris,Widenmeyer, Markus,Grasser, Stefan,Koehler, Klaus,Anwander, Reiner
, p. 1544 - 1545 (2000)
-
Fluorescence spectroscopy of aromatic species produced in rich premixed ethylene flames
Ciajolo,Ragucci,Apicella,Barbella,De Joannon,Tregrossi
, p. 835 - 841 (2001)
The fluorescence spectra of the condensed species (CS) collected in the soot inception region of a rich premixed laminar ethylene/oxygen flame have been measured by excitation in the UV at 266 and 355 nm excitation wavelength. The contribution of the most abundant polycyclic aromatic hydrocarbons (PAH) to the CS fluorescence has been evaluated in order to attribute the CS fluorescence at different emission wavelengths to specific aromatic structures. The fluorescence peaks detected in the UV region of the CS fluorescence spectrum was found to be mainly due to a typical PAH like fluorene, that is, the most fluorescent one among the PAH analyzed in the CS by chromatographic analysis, The CS exhibited the larger emission in the visible where the PAH contribution has been shown to be negligible and other fluorescing aromatic species, not identified by chromatographic analysis of the CS, have to be considered responsible for the visible fluorescence. Laser induced fluorescence (LIF) flame measurements excited at 266 nm and detected at two selected wavelengths (310 and 410 nm) have been performed along the flame axis and compared with the CS fluorescence intensity. The LIF and CS fluorescence signals show quite similar axial trends demonstrating that the LIF signals are related to CS fluorescence. In particular, the LIF fluorescence signals detected in the UV could be attributed to the PAH fluorescence whereas the unidentified species contained in the CS can be followed by LIF detection in the visible region.
Palladium-Catalyzed Coupling of Biphenyl-2-yl Trifluoromethanesulfonates with Dibromomethane to Access Fluorenes
Pan, Shulei,Zhang, Yanghui,Zhu, Qiongqiong
, (2022/03/27)
A facile and efficient method has been developed for the synthesis of fluorenes by Pd-catalyzed C-H alkylation of biphenyl-2-yl trifluoromethanesulfonates. The trifluoromethanesulfonates are more readily available and more environmentally benign than biphenyl iodides, and are advantageous substrates for traceless directing-groupassisted C-H activation. The reaction generates C,C-palladacycles as the key intermediates that form two C(sp2)-C(sp3) bonds through reaction with CH2Br2. The reaction tolerates various functional groups, permitting easy access to a range of fluorene derivatives.
Pd-Catalyzed Assembly of Fluoren-9-ones by Merging of C-H Activation and Difluorocarbene Transfer
Liu, Xiaobing,Sheng, Heyun,Zhou, Yao,Song, Qiuling
supporting information, p. 2543 - 2547 (2021/05/05)
We disclose a novel Pd-catalyzed assembly of fluoren-9-ones by merging of C-H activation and difluorocarbene transfer. ClCF2COONa served as a difluorocarbene precursor to be harnessed as a carbonyl source in this transformation. The current protocol enables us to afford fluoren-9-ones in high yields with excellent functional group compatibility, which also represents the first example of using difluorocarbene as a coupling partner in transition-metal-catalyzed C-H activation.
Method for reducing carbonyl reduction to methylene under illumination
-
Paragraph 0033-0038, (2021/09/29)
The invention belongs to the technical field of organic chemical synthesis. The method comprises the following steps: (1) mixing the carbonyl compound and the amine compound in a solvent, reacting 3 - 6 under the illumination of 380 - 456 nm, the reaction system is low in toxicity, high in atom utilization rate 12 - 24h. and production efficiency, safe and controllable in reaction process and capable of simplifying the operation in the preparation and production process. At the same time, the residue toxicity of the reaction is minimized, the pollution caused by the production process to the environment is reduced, and the steps and operations of removing residues after the reaction are simplified. In addition, the reactant feedstock is readily available. The reactant does not need additional modification before the reaction, can be directly used for preparing production, simplifies the operation steps, and shortens the reaction route. The production cost is obviously reduced.
A facile and versatile electro-reductive system for hydrodefunctionalization under ambient conditions
Huang, Binbin,Guo, Lin,Xia, Wujiong
supporting information, p. 2095 - 2103 (2021/03/26)
A general electrochemical system for reductive hydrodefunctionalization is described, employing the inexpensive and easily available triethylamine (Et3N) as a sacrificial reductant. This protocol is characterized by facile operation, sustainable conditions, and exceptionally wide substrate scope covering the cleavage of C-halogen, N-S, N-C, O-S, O-C, C-C and C-N bonds. Notably, the selectivity and capability of reduction can be conveniently switched by simple incorporation or removal of an alcohol as a co-solvent.