Naringin literature
Chiral separation of hesperidin and naringin and its analysis in a butanol extract of Launeae arborescens
Belboukhari, Nasser,Cheriti, Abdelkrim,Roussel, Christian,Vanthuyne, Nicolas
, p. 669 - 681 (2010)
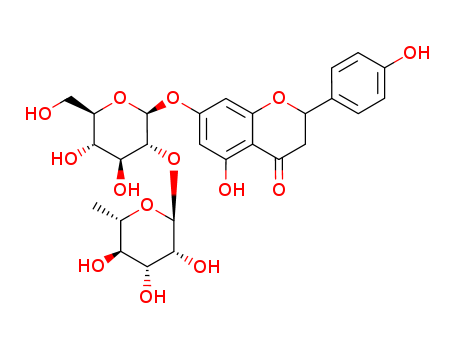
Two flavanone glycosides were isolated from the aerial part of Launeae arborescens (Asteraceae), which were identified as hesperidin and naringin. They are the most abundant flavonoids in the edible parts of many species of citrus fruits. In this study, we were interested in the chiral separation and determination of the diastereomerisation barriers of hesperidin and naringin by HPLC methods. The chiral separation HPLC screening of diastereomers of hesperidin and naringin by HPLC methods was accomplished in the normal-phase mode using 11 chiral stationary phases and various n-hexane/alcohol mobile phases. The rate constants and activation energy of diastereomerisation (G#) of flavanones, naringin and hesperidin were determined, respectively, on Chiralpak IC and Chiralpak IA. The analysis of flavanones isolated in butanol extracts of Launeae arborescens were confirmed by HPLC on Chiralpak IC.
Preparation and evaluation of a triazole-bridged bis(β-cyclodextrin)–bonded chiral stationary phase for HPLC
Shuang, Yazhou,Liao, Yuqin,Wang, Hui,Wang, Yuanxing,Li, Laisheng
, p. 168 - 184 (2019/11/25)
A triazole-bridged bis(β-cyclodextrin) was synthesized via a high-yield Click Chemistry reaction between 6-azido-β-cyclodextrin and 6-propynylamino-β-cyclodextrin, and then it was bonded onto ordered silica gel SBA-15 to obtain a novel triazole-bridged bis (β-cyclodextrin)–bonded chiral stationary phase (TBCDP). The structures of the bridged cyclodextrin and TBCDP were characterized by the infrared spectroscopy, mass spectrometry, elemental analysis, and thermogravimetric analysis. The chiral performance of TBCDP was evaluated by using chiral pesticides and drugs as probes including triazoles, flavanones, dansyl amino acids and β-blockers. Some effects of the composition in mobile phase and pH value on the enantioseparations were investigated in different modes. The nine triazoles, eight flavanones, and eight dansyl amino acids were successfully resolved on TBCDP under the reversed phase with the resolutions of hexaconazole, 2′-hydroxyflavanone, and dansyl-DL-tyrosine, which were 2.49, 5.40, and 3.25 within 30 minutes, respectively. The ten β-blockers were also separated under the polar organic mode with the resolution of arotinolol reached 1.71. Some related separation mechanisms were discussed preliminary. Compared with the native cyclodextrin stationary phase (CDSP), TBCDP has higher enantioselectivity to separate more analytes, which benefited from the synergistic inclusion ability of the two adjacent cavities and bridging linker of TBCDP, thereby enabling it a promising prospect in chiral drugs and food analysis.
Enthalpy-entropy compensation effect in the chalcone formation from naringin in water-ethanol mixtures
Gonzalez, Evangelina A.,Nazareno, Monica A.,Borsarelli, Claudio D.
, p. 2052 - 2056 (2007/10/03)
The isomerisation equilibrium between the flavanone and chalcone forms of the naturally occurring flavonoid naringin (7-rhamnoglucosyl-4′,5-dihydroxyflavanone) has been studied in water-ethanol mixtures in the presence of NaOH. The variation of the observed pseudo-first order rate constant for the equilibrium reaction, kobs, and the equilibrium composition were determined as a function of the base concentration (-4.0 < log[NaOH] < - 2.4) and the water molar fraction (0.03 ≤ Xw ≤ 1.00) of the solvent mixture. The variation of the ring opening kop, and the cyclisation kcy, rate constants with the base concentration and solvent composition indicated that the isomerisation reaction is mediated by a carbanion intermediate. The temperature effect on kop and kcy showed that only the activation enthalpy and entropy changes for the ring-opening reaction, ΔHop? and ΔSop?, were dependent on the solvent composition. In fact, a good linear correlation of a plot of ΔHop? vs. ΔSop?, indicate the existence of an enthalpy-entropy compensation effect. This result was associated with changes in the balance of hydrogen-bonding interactions between the intermediate carbanion and its solvation sphere as solvent composition was modified.
Antimicrobial compositions of matter and a process for preparing antimicrobial compositions of matter from naturally occurring flavanoid glycosides
-
, (2008/06/13)
A process for the production of antimicrobial compositions from naturally occurring flavanoid glycosides. Flavanoid glycosides are acid hydrolyzed under substantially quiescent condition at a temperature within the range of about 60° C. and about 100° C. for a sufficient time to hydrolyze the flavanoid glycoside to partially hydrolyzed flavanoid compositions having antimicrobial activity.