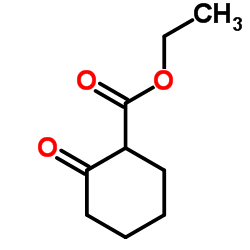
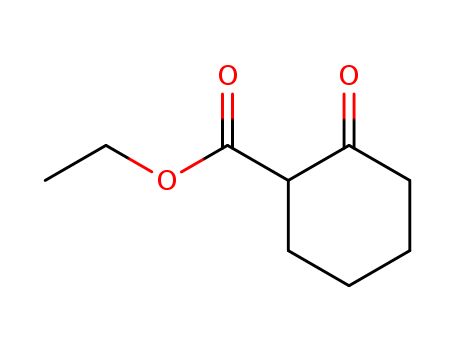
Ethyl 2-oxocyclohexanecarboxylate literature
Facile carbethoxylation and carbamoylation of ketones
Pazdera, Pavel,Simbera, Jan
, p. 297 - 301 (2011)
-
-
Torii,S. et al.
, p. 293 - 294 (1978)
-
-
Stork et al.
, p. 2029 (1954)
-
-
Zau'yalov,Kochanova
, (1978)
-
Phosphate tricyclic coumarin analogs as steroid sulfatase inhibitors: Synthesis and biological activity
Kozak, Witold,Dasko, Mateusz,Maslyk, Maciej,Pieczykolan, Jerzy S.,Gielniewski, Bartlomiej,Rachon, Janusz,Demkowicz, Sebastian
, p. 44350 - 44358 (2014)
In the present work, we report convenient methods for the synthesis and biological evaluation of phosphate tricyclic coumarin derivatives as potential steroid sulfatase inhibitors. The described synthesis includes the straightforward preparation of 7-hydroxy-2,3-dihydro-1H-cyclopenta[c]chromen-4-one, 3-hydroxy-7,8,9,10-tetrahydro-6H-benzo[c]chromen-6-one and 3-hydroxy-8,9,10,11-tetrahydro-7H-cyclohepta[c]chromen-6-one modified with various phosphate moieties. The inhibitory effects of the synthesized compounds were tested on STS isolated from human placenta as well as the MCF-7, MDA-MB-231 and MDA-MB-435S cancer cell lines. Most of the new STS inhibitors possessed IC50 values between 21 to 159 μM. In the course of our investigation, the largest inhibitory effects in the STS enzyme assays were observed for the three compounds 9p, 9r and 9s, with IC50 values of 36.4, 37.8 and 21.5 μM, respectively (IC50 value of 1.0 μM for the 665-COUMATE used as a reference). The compound 9r, exhibited the highest potency against MCF-7, an estrogen receptor positive (ER+) cell line, with a GI50 value of 24.7 μM. The structure-activity relationships of the synthesized coumarin derivatives with the STS enzyme are discussed.
Solvent-free Dieckmann condensation reactions of diethyl adipate and pimelate
Toda, Fumio,Suzuki, Takaaki,Higa, Satoru
, p. 3521 - 3522 (1998)
Dieckmann condensation reactions of diethyl adipate and pimelate proceeded efficiently in the absence of solvent, and the reaction products were collected by a direct distillation from the solvent-free reaction mixture.
Enantioselective α-Amination of Acyclic 1,3-Dicarbonyls Catalyzed by N-Heterocyclic Carbene
Santra, Surojit,Maji, Ujjwal,Guin, Joyram
supporting information, p. 468 - 473 (2020/02/04)
Herein, we describe a method for the catalytic enantioselective α-amination of α-substituted acyclic 1,3-ketoamides and 1,3-amidoesters that affords the products possessing N-substituted quaternary stereocenters with a chiral N-heterocyclic carbene (NHC). The reaction is based on the utilization of an intrinsic Br?nsted base characteristic of NHC that enables the catalytic formation of a chiral ion pair comprising the enolate and the azolium ion. A series of challenging open-chain α-substituted 1,3-dicarbonyls are aminated via this method with ee's of ≤99%.
Radical Aza-Cyclization of α-Imino-oxy Acids for Synthesis of Alkene-Containing N-Heterocycles via Dual Cobaloxime and Photoredox Catalysis
Tu, Jia-Lin,Liu, Jia-Li,Tang, Wan,Su, Ma,Liu, Feng
supporting information, p. 1222 - 1226 (2020/02/15)
Nitrogen-containing heterocycles are prevalent in both naturally and synthetically bioactive molecules. We report herein an unprecedented protocol for radical aza-cyclization of α-imino-oxy acids with pendant alkenes via synergistic photoredox and cobaloxime catalysis. With or without alkenes as the intermolecular cross-coupling partners, the transformation provides a variety of corresponding alkene-containing dihydropyrrole products in satisfactory yields. In the presence of external alkenes, the tandem reaction generates E-selective coupling products with excellent chemo- and stereoselectivity.
Coumarin-dithiocarbamate hybrids as novel multitarget AChE and MAO-B inhibitors against Alzheimer's disease: Design, synthesis and biological evaluation
He, Qi,Liu, Jing,Lan, Jin-Shuai,Ding, Jiaoli,Sun, Yongbing,Fang, Yuanying,Jiang, Neng,Yang, Zunhua,Sun, Liyuan,Jin, Yi,Xie, Sai-Sai
supporting information, p. 512 - 528 (2018/09/29)
A series of new coumarin-dithiocarbamate hybrids were designed and synthesized as multitarget agents for the treatment of Alzheimer's disease. Most of them showed potent and clearly selective inhibition towards AChE and MAO-B. Among these compounds, compound 8f demonstrated the most potent inhibition to AChE with IC50 values of 0.0068 μM and 0.0089 μM for eeAChE and hAChE, respectively. Compound 8g was identified as the most potent inhibitor to hMAO-B, and it is also a good and balanced inhibitor to both hAChE and hMAO-B (0.114 μM for hAChE; 0.101 μM for hMAO-B). Kinetic and molecular modeling studies revealed that 8g was a dual binding site inhibitor for AChE and a competitive inhibitor for MAO-B. Further studies indicated that 8g could penetrate the BBB and exhibit no toxicity on SH-SY5Y neuroblastoma cells. More importantly, 8g did not display any acute toxicity in mice at doses up to 2500 mg/kg and could reverse the cognitive dysfunction of scopolamine-induced AD mice. Overall, these results highlighted 8g as a potential multitarget agent for AD treatment and offered a starting point for design of new multitarget AChE/MAO-B inhibitors based on dithiocarbamate scaffold.
Cobalt-Catalyzed Cross-Couplings between Alkenyl Acetates and Aryl or Alkenyl Zinc Pivalates
Li, Jie,Knochel, Paul
supporting information, p. 11436 - 11440 (2018/08/11)
CoBr2 (5 mol %) in the presence of 2,2′-bipyridyl (5 mol %) enables electrophilic alkenylations between easily accessible alkenyl acetates or tosylates and various functionalized aryl zinc pivalates at ambient temperature. This cobalt-catalyzed