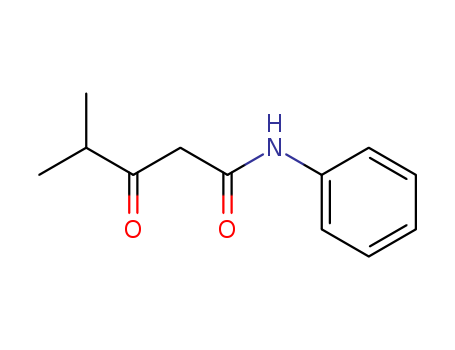
N-Phenyl-isobutyloylacetamide literature
α-Unsubstituted Pyrroles by NHC-Catalyzed Three-Component Coupling: Direct Synthesis of a Versatile Atorvastatin Derivative
Fleige, Mirco,Glorius, Frank
, p. 10773 - 10776 (2017)
A practical one-pot cascade reaction protocol provides direct access to valuable 1,2,4-trisubstituted pyrroles. The process involves an N-heterocyclic carbene (NHC)-catalyzed Stetter-type hydroformylation using glycolaldehyde dimer as a novel C1 building-block, followed by a Paal-Knorr condensation with primary amines. The reaction makes use of simple and commercially available starting-materials and catalyst, an important feature regarding applicability and utility. Low catalyst loading under mild reaction conditions afforded a variety of 1,2,4-substituted pyrroles in a transition-metal-free reaction with high step economy and good yields. This methodology is applied in the synthesis of a versatile Atorvastatin precursor, in which a variety of modifications at the pyrrole core structure are possible.
An efficient synthesis of highly substituted pyrrole and bis pyrrole derivatives
Sagyam, Rajeshwar Reddy,Vurimidi, Himabindu,Padi, Pratap Reddy,Ghanta, Mahesh Reddy
, p. 923 - 926 (2007)
(Chemical Equation Presented) An efficient synthesis of highly substituted pyrrole and bis pyrrole derivatives is reported.
Ru-NHC-Catalyzed Asymmetric Hydrogenation of 2-Quinolones to Chiral 3,4-Dihydro-2-Quinolones
Daniliuc, Constantin,Glorius, Frank,Hu, Tianjiao,Lückemeier, Lukas
supporting information, p. 23193 - 23196 (2021/09/25)
Direct enantioselective hydrogenation of unsaturated compounds to generate chiral three-dimensional motifs is one of the most straightforward and important approaches in synthetic chemistry. We realized the Ru(II)-NHC-catalyzed asymmetric hydrogenation of 2-quinolones under mild reaction conditions. Alkyl-, aryl- and halogen-substituted optically active dihydro-2-quinolones were obtained in high yields with moderate to excellent enantioselectivities. The reaction provides an efficient and atom-economic pathway to construct simple chiral 3,4-dihydro-2-quinolones. The desired products could be further reduced to tetrahydroquinolines and octahydroquinolones.
Atorvastatin Derived HMG-CoA Reductase Degradation Inducing Compound
-
Paragraph 0137; 0364; 0380-0382, (2020/12/01)
The present invention relates to a HMG-CoA reductase degradation-inducing compound and, more specifically, to a bifunctional compound in which atorvastatin and E3 ubiquitin ligase binding moiety are chemically linked as HMG-CoA reductase binding moiety, to a production method thereof, to a HMG-CoA reductase degradation method using the same, and to a pharmaceutical composition for preventing or treating HMG-CoA reductase-related diseases, comprising the same.
Preparation method of atorvastatin calcium isomers
-
Paragraph 0022-0034; 30051-0052, (2018/09/11)
The invention relates to a preparation method of atorvastatin calcium isomers [R-(R*,R*)]-2-(3-fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl)-2-phenyl-4-[(aniline)carbonyl]-1H-pyrrole-1-calciumenanthate salt (IMP-1 for short) and [R-(R*,R*)]-3-(2-fluorophenyl)-beta, delta-dihydroxyl -5-(1-Methylethyl)-3-phenyl-4-[(anilino)carbonyl]-1H-pyrrole-1- calcium enanthate salt (IMP-2 for short). According to the preparation method, a preparation method of a reference substance is provided for the quality research of drugs, and an important guiding significance is provided for the safe medicationof atorvastatin calcium.
Preparation method of atorvastatin calcium
-
, (2018/07/30)
The invention belongs to the technical field of medicine preparation and particularly relates to a patent application about a novel preparation method of atorvastatin calcium. The method includes steps of preparing intermediates including: 2-methyl-3-carbonyl-methyl pentanoate, 2-methyl-3,5-dicarbonyl-5-anilino-butane, 4-methyl-3-oxo-N-phenyl-2-benzylidene pentanamide, 4-(4-fluorophenyl)-2-(2-methylpropionyl)-4-oxo-N-beta-diphenyl butyrylamide. The preparation method employs cheap and easy-to-obtained raw materials, has simple reactions and operations, and has great industrial application prospect. In conclusion, the preparation method has high reaction efficiency and product yield, is good in repeatability, is suitable for industrial production and has great application value and promotion and application significance.