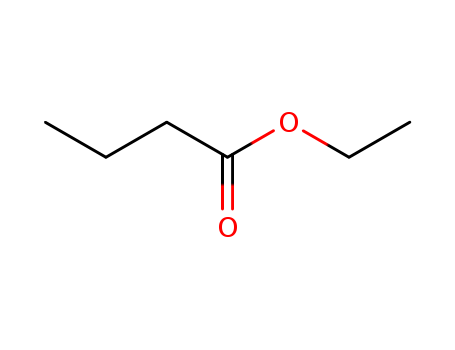
Ethyl butyrate literature
Lipase-catalyzed synthesis of ethyl hexanoate in microemulsion system
Tan, Zhongqin,Han, Xiaoxiang,Hu, Xiaoli,Du, Huan,Bao, Xiuxiu
, p. 9675 - 9678 (2013)
This paper studied lipase-catalyzed synthesis of ethyl hexanoate in dodecylbenzenesulfonic acid/isooctane/water microemulsion system. The effect of several parameters, such as w0 ([H2O]/[surfactant]) value, reaction time, reaction temperature, oil phase solvent, buffer solution pH value of microemulsion system on the esterification have been investigated. The results showed that the best experimental conditions for catalytic synthesis ethyl hexanoate were as follows: w0 = 4, reaction time 4 h, reaction temperature 40 °C, buffer solution pH 7. Under these conditions, the conversion of ethyl hexanoate can reach 98.5 %. Lipase-catalyzed synthesis of ethyl hexanoate in dodecylbenzenesulfonic acid inverse microemulsion system has triple mechanism, namely acid catalyzes, microemulsion catalyzes and enzyme catalyzes.
Triethylborane-induced radical reactions with gallium- and indium hydrides
Takami, Kazuaki,Mikami, Satoshi,Yorimitsu, Hideki,Shinokubo, Hiroshi,Oshima, Koichiro
, p. 6627 - 6635 (2003)
A gallium hydride reagent, HGaCl2, was found to act as a radical mediator. Treatment of alkyl halides with the gallium hydride reagent, generated from gallium trichloride and sodium bis(2-methoxyethoxy)aluminum hydride, provided the corresponding reduced products in excellent yields. Radical cyclization of halo acetals was also successful with not only the stoichiometric gallium reagent but also a catalytic amount of gallium trichloride combined with stoichiometric aluminum hydride as a hydride source. An indium hydride reagent, HInCl2, prepared from indium trichloride and diisobutylaluminum hydride also worked as a radical mediator. HInCl2 could reduce aryl iodides and bromides in the presence of Et3B as a radical initiator.
The combined use of ultrasound and molecular sieves improves the synthesis of ethyl butyrate catalyzed by immobilized Thermomyces lanuginosus lipase
Paludo, Natalia,Alves, Joana S.,Altmann, Cintia,Ayub, Marco A.Z.,Fernandez-Lafuente, Roberto,Rodrigues, Rafael C.
, p. 89 - 94 (2015)
In this work, the combined use of ultrasound energy and molecular sieves was investigated for the synthesis of ethyl butyrate, ester with mango and banana notes, catalyzed by the immobilized lipase from Thermomyces lanuginosus (Lipozyme TL-IM). Initially, the best concentrations of biocatalysts (35%) and butyric acid (0.7 M) were tested using ultrasound as an alternative to mechanical agitation. The amount of acid in the reaction could be increased by 2-fold when compared to previous works where mechanical agitation was used. In the next step, substrate molar ratio and reaction temperature were optimized and the best conditions were at their lowest levels: 1:1 (acid:alcohol), and 30 °C, reaching 61% of conversion in 6 h. Molecular sieves (3 A?) were added to optimized reaction medium in order to remove the formed water and improve the maximum yield. The reaction yield increased 1.5 times, reaching 90% of conversion in 6 h, when 60 mg of molecular sieves per mmol of butyric acid was used. Finally, the reuse of Lipozyme TL-IM for the ultrasound-assisted synthesis of ethyl butyrate was verified for 10 batches, without any appreciable loss of activity, whereas in systems using mechanical agitation, the biocatalyst was completely inactivated after 5 batches. These results suggest that the combined use of ultrasound and molecular sieves greatly improve esterification reactions by stabilizing the enzyme and increasing yields.
-
Larock
, p. 834 (1974)
-
New possibilities in the synthesis of fuel oxygenates from renewable sources
Varfolomeev,Vol’eva,Komissarova,Kurkovskaya,Malkova,Ovsyannikova,Gumerov,Usmanov
, p. 717 - 724 (2019)
A general problem in the production of the main types of liquid biofuel, bioethanol and biodiesel, is that renewable resources are not utilized completely. These are ballast polyols: hemicellulose or its structural units, pentaatomic monosaccharides (xylose and arabinose), and biodiesel glycerol. The problem of utilization of these compounds by transforming them into a hydrophobized fuel form by the conversion to cyclic ketals (CK) during condensation with lower carbonyl compounds is reviewed. The CK—ethanol pair significantly increases the octane number and provides phase stability of fuel compositions. The ability of CK to inhibit radical processes responsible for fuel characteristics was studied in model reactions with phenyl radicals and atomic chlorine. The carbon-centered radicals formed in protic media are transformed into more stable cyclic radical cations. Alternative methods of processing natural raw materials using biocatalysis and supercritical fluid technologies are analyzed.
-
Huyser et al.
, p. 4377 (1965)
-
Fluorescent microplate assay method for high-throughput detection of lipase transesterification activity
Zheng, Jianyong,Wei, Wei,Lan, Xing,Zhang, Yinjun,Wang, Zhao
, p. 26 - 28 (2018)
This study describes a sensitive and fluorescent microplate assay method to detect lipase transesterification activity. Lipase-catalyzed transesterification between butyryl 4-methyl umbelliferone (Bu-4-Mu) and methanol in tert-butanol was selected as the model reaction. The release of 4-methylumbelliferone (4-Mu) in the reaction was determined by detecting the fluorescence intensity at λex 330 nm and λem 390 nm. Several lipases were used to investigate the accuracy and efficiency of the proposed method. Apparent Michaelis constant (Km) was calculated for transesterification between Bu-4-Mu and methanol by the lipases. The main advantages of the assay method include high sensitivity, inexpensive reagents, and simple detection process.
-
Filler,R.,Naqvi,S.M.
, p. 879 - 889 (1963)
-
Zinc Complexes with Cyanoxime: Structural, Spectroscopic, and Catalysis Studies in the Pivaloylcyanoxime-Zn System
Opalade, Adedamola A.,Karmakar, Anirban,Rúbio,Pombeiro, Armando J. L.,Gerasimchuk, Nikolay
, p. 13962 - 13974 (2017)
Reaction of 2-hydroxyimino-4,4-dimethyl-3-oxo-pentanenitrile (common abbreviation HPiCO, pivaloyl-cyanoxime) with zinc sulfate in an aqueous solution results in the formation of the two new complexes: [Zn(PiCO){H(PiCO)2}(H2O)] (I) and tetranuclear Zn complex [Zn4(μ3-OH)2(PiCO)6 (H2O)4] (II). Both complexes were characterized by elemental analysis, IR- and UV-visible spectra, DSC/TGA studies, and X-ray analysis. In complex II, the PiCO- cyanoxime anion adopts three bidentate binding modes: O-monodentate, chelating (κ2), and bridging (2) coordinations. Also, the ligand represents the mixture of two diasteromers (cis-anti and cis-syn) that form five- and six-membered chelate rings with Zn atoms and cocrystallize in one unit cell at population of 0.57-0.43. There are two crystallographically different Zn-centers in the ASU, and two μ3-bridging hydroxo-groups arrange via inversion center the formation of an elegant tetranuclear complex. Each Zn atom has a molecule of coordinated water and is in the distorted octahedral environment. Because of the structural flexibility and multidentate propensity of the pivaloyl-cyanoxime, complex II may act as a structural model of naturally occurring Zn-containing enzymes. Indeed, compound I exhibits an efficient catalytic performance for transesterification reaction of various esters in ethanol under mild reaction conditions. Therefore, obtained results allow assignment of observed activity as green catalysis.
Poly(vinylsulfonic acid)-grafted solid catalysts: New materials for acid-catalysed organic synthetic reactions
Okayasu, Teruyuki,Saito, Kei,Nishide, Hiroyuki,Hearn, Milton T. W.
, p. 1981 - 1989 (2010)
The synthesis, characterisation and application of novel high-density poly(vinylsulfonic acid)-grafted solid acid catalysts are described. A graft, radical polymerization procedure was employed, allowing the immobilisation of the acid form of vinylsulfonic acid monomer onto various carrier materials, such as polystyrene, silica or polysaccharide-based gels. The highest acid-exchange capacity (as determined by acid-base titration methods) achieved with these new materials was 5.2 mmol H+ g-1. The properties of these PVS-grafted materials as solid state acid catalysts have been examined from several perspectives, including their fundamental properties as materials with extremely high acid dissociation characteristics, their structural features as revealed from IR and solid-state NMR measurements, their thermal stability properties, and their surface morphologies, humidity dependencies and functionality. Compared to many other types of acid catalysts, these high-density poly(vinylsulfonic acid)-grafted materials demonstrated superior catalytic performance in esterification, Friedel-Crafts acylation, and condensation reactions. Moreover, these novel materials show high stability, significant anticorrosion capability and can be easily recycled. The Royal Society of Chemistry 2010.
Selective reduction of α,β-unsaturated esters with NaBH4-BiCl3 system
Ren,Pan,Dong,Wu
, p. 3395 - 3399 (1995)
Sodium borohydride-bismuth chloride system was applied for the selective reduction of carbon-carbon double bond of α,β-unsaturated esters with high selectivity.
-
Tundo
, p. 2048 (1979)
-
-
Sumrell,Ham
, p. 5573 (1956)
-
-
Chi-San Chen
, p. 213,215 (1978)
-
Efficient and catalyst-free condensation of acid chlorides and alcohols using continuous flow
Van Waes, Frederik E. A.,Cukalovic, A.,Stevens, Christian V.,Drabowicz, J.
, p. 2776 - 2779,4 (2012)
An efficient, catalyst-free continuous flow procedure for the condensation of acid chlorides and alcohols was developed. Different esters could be obtained using this protocol with excellent conversions starting from the corresponding acid chlorides and alcohols in very short reaction times (5-7 min). The reaction was performed solventless for liquid reagents but requires a solvent for solid reagents in order to prevent clogging of the microreactor. Since no catalyst is needed, the purification of the reaction mixture is very straightforward. Scale-up of the reaction to a microreactor with an internal volume of 13.8 ml makes it possible to produce 2.2 g min-1 of ester with an isolated yield of 98% and recuperation of the formed HCl.
Ultrasound technology and molecular sieves improve the thermodynamically controlled esterification of butyric acid mediated by immobilized lipase from Rhizomucor miehei
Fallavena, Lucas P.,Antunes, Fabio H. F.,Alves, Joana S.,Paludo, Natalia,Ayub, Marco A. Z.,Fernandez-Lafuente, Roberto,Rodrigues, Rafael C.
, p. 8675 - 8681 (2014)
In this research, the effects of ultrasound stirring and the addition of molecular sieves on esterification reactions between butyric acid and several alcohols catalyzed by immobilized lipase from Rhizomucor miehei (Lipozyme RM-IM) were studied. Among the tested alcohols, 1-propanol and isobutanol allowed the highest activities, whereas Lipozyme RM-IM showed poor activities for esterification using secondary and tertiary alcohols. Different solvents were also tested and n-hexane was selected because of its reaction effects, besides being cheaper, available at low boiling point, and ease of recovery. Using the preselected alcohol and solvent, other reaction parameters (butyric acid concentration, temperature, substrate molar rate, and biocatalyst content) were studied to optimize the reaction conditions. Optimal conditions were acid concentration, 0.7 M; substrate molar ratio, 11 alcohol-acid; temperature 45 °C; biocatalyst content, 14% (by substrate mass). Under these conditions, it was possible to obtain a yield of 86% of butyl butyrate in 2.5 h. When molecular sieves (90 mg mmol-1 butytic acid) were added to the reaction, the observed yield increased to 96%. The biocatalyst was used in 5 successive reaction cycles keeping 100% of its initial activity. The overall process productivity was improved 2-fold when compared to the traditional mechanical agitation, showing that ultrasound is a promising technology for application in biocatalysis. The Royal Society of Chemistry.
Biocatalytic production of ethyl butyrate from butyric acid with immobilized Candida rugosa lipase on cotton cloth
Shu, Chengliang,Cai, Jin,Huang, Lei,Zhu, Xiangcheng,Xu, Zhinan
, p. 139 - 144 (2011)
A novel method involving polyethylenimine (PEI) coating and glutaraldehyde cross-linking processes was developed to immobilize Candida rugosa lipase onto cotton cloth. After the systematic investigation, the optimal lipase immobilization was achieved when 0.1 g lipase was loaded on 1.5 g support, which was pretreated with 10 ml of 1.0 mg/ml PEI solution at pH 8.0. Subsequent catalytic analysis of immobilized lipase for ethyl butyrate synthesis was also carried out in the Erlenmeyer flasks. The results indicated that when optimal 0.25 M ethanol and 0.6 M butyric acid were catalyzed by the immobilized lipase at 25 °C, the highest conversion yield of 91.2% and 1.27 mmol h-1 g-1 productivity of ethyl butyrate were obtained. Furthermore, a kinetic model of Ping Pong Bi-Bi mode with inhibition of both substrates was proposed and validated by experimental data. To explore the practical potential of immobilized lipase, its operational stability was evaluated and the residual activity was remained about 50% after 12 repeated recycles, with a half-life time of about 300 h for the immobilized lipase. Finally, a recycle batch reactor using immobilized lipase was developed for ethyl butyrate production. The achieved result of 0.85 M final ethyl butyrate concentration, with the conversion of 70.6% and the productivity of 1.45 mmol h-1 g -1, had revealed the promising potential of this immobilized lipase in practical applications.
Liquid phase esterification of levulinic acid into ethyl levulinate over sulphobenzylated nanoporous Al-SBA-15 catalyst
Kumaravel, Sakthivel,Thiripuranthagan, Sivakumar,Radhakrishnan, Ramakrishnan,Erusappan, Elangovan,Durai, Mani,Devarajan, Arulselvan,Mukannan, Arivanandhan
, p. 6965 - 6977 (2019)
Value added chemicals, fuels, and fuel additives can be obtained from cheap bio masses such as levulinic acid. Levulinic acid is the dehydration and hydrolysis products of pentoses and hexoses. The present work deals with the synthesis of sulphobenzylated Al-SBA-15, [SO3H-Bz-Al-SBA-15], characterization by various analytical techniques such as XRD, BET, FT-IR, TGA, DTA, FE-SEM/EDS and HR-TEM/EDX techniques and evaluation of catalytic activity towards esterification of levulinic acid to ethyl levulinate under mild and non corrosive conditions. Sulphonation of the aromatic ring of the benzyl group has been done in different amounts to get nanoporous x% SO3H-Bz-Al-SBA-15 catalysts where (x = 0.02, 0.04, 0.06, 0.08 and 0.10% w/w). Among them 0.08% SO3H-Bz-Al-SBA-15 catalyst showed the highest conversion of levulinic acid (100%) with the highest selectivity towards ethyl levulinate (100%). Esterification of levulinic acid has been carried out with different primary alcohols and all of them yielded 100% selectivity towards alkyl levulinate. However conversion level of levulinic acid was found to be different with different alcohols. Reaction conditions have been optimized. The results were compared with other supported catalysts and discussed.
-
Kutz,Adkins
, p. 4391,4397 (1930)
-
Reactivity of a Carbon-Supported Single-Site Molybdenum Dioxo Catalyst for Biodiesel Synthesis
Mouat, Aidan R.,Lohr, Tracy L.,Wegener, Evan C.,Miller, Jeffrey T.,Delferro, Massimiliano,Stair, Peter C.,Marks, Tobin J.
, p. 6762 - 6769 (2016)
A single-site molybdenum dioxo catalyst, (Oc)2Mo(=O)2@C, was prepared via direct grafting of MoO2Cl2(dme) (dme = 1,2-dimethoxyethane) on high-surface-area activated carbon. The physicochemical and chemical properties of this catalyst were fully characterized by N2 physisorption, ICP-AES/OES, PXRD, STEM, XPS, XAS, temperature-programmed reduction with H2 (TPR-H2), and temperature-programmed NH3 desorption (TPD-NH3). The single-site nature of the Mo species is corroborated by XPS and TPR-H2 data, and it exhibits the lowest reported MoOx Tmax of reduction reported to date, suggesting a highly reactive MoVI center. (Oc)2Mo(=O)2@C catalyzes the transesterification of a variety of esters and triglycerides with ethanol, exhibiting high activity at moderate temperatures (60-90 °C) and with negligible deactivation. (Oc)2Mo(=O)2@C is resistant to water and can be recycled at least three times with no loss of activity. The transesterification reaction is determined experimentally to be first order in [ethanol] and first order in [Mo] with Δ;H? = 10.5(8) kcal mol-1 and Δ;S? = -32(2) eu. The low energy of activation is consistent with the moderate conditions needed to achieve rapid turnover. This highly active carbon-supported single-site molybdenum dioxo species is thus an efficient, robust, and low-cost catalyst with significant potential for transesterification processes.
The preparation of homogeneous triglycerides of eicosapentaenoic acid and docosahexaenoic acid by lipase
Haraldsson, Gudmundur G.,Gudmundsson, Birgir Oe.,Almarsson, Oern
, p. 5791 - 5794 (1993)
The highly efficient generation of homogeneous triglycerides of either pure eicosapentaenoic acid, 1, or docosahexaenoic acid, 2, by an immobilized nonregiospecific yeast lipase from Candida antarctica is described.
Effect of the Presence of Surfactants and Immobilization Conditions on Catalysts’ Properties of Rhizomucor miehei Lipase onto Chitosan
de Oliveira, Ulisses M. F.,Lima de Matos, Leonardo J. B.,de Souza, Maria Cristiane M.,Pinheiro, Bruna B.,dos Santos, José C. S.,Gon?alves, Luciana R. B.
, p. 1263 - 1285 (2018)
Lipase from Rhizomucor miehei (RML) was immobilized onto chitosan support in the presence of some surfactants added at low levels using two different strategies. In the first approach, the enzyme was immobilized in the presence of surfactants on chitosan supports previously functionalized with glutaraldehyde. In the second one, after prior enzyme adsorption on chitosan beads in the presence of surfactants, the complex chitosan beads-enzyme was then cross-linked with glutaraldehyde. The effects of surfactant concentrations on the activities of free and immobilized RML were evaluated. Hexadecyltrimethylammonium bromide (CTAB) promoted an inhibition of enzyme activity while the nonionic surfactant Triton X-100 caused a slight increase in the catalytic activity of the free enzyme and the derivatives produced in both methods of immobilization. The best derivatives were achieved when the lipase was firstly adsorbed on chitosan beads at 4?°C for 1?h, 220?rpm followed by cross-link the complex chitosan beads-enzyme with glutaraldehyde 0.6% v.v?1 at pH 7. The derivatives obtained under these conditions showed high catalytic activity and excellent thermal stability at 60° and 37?°C. The best derivative was also evaluated in the synthesis of two flavor esters namely methyl and ethyl butyrate. At non-optimized conditions, the maximum conversion yield for methyl butyrate was 89%, and for ethyl butyrate, the esterification yield was 92%. The results for both esterifications were similar to those obtained when the commercial enzyme Lipozyme and free enzyme were used in the same reaction conditions and higher than the one achieved in the absence of the selected surfactant.
-
Feuer,H. et al.
, p. 3622 - 3625 (1968)
-
Ruthenium Complex-Catalyzed Silylation of Olefins. Selective Synthesis of Allylsilanes
Hori, Yoji,Mitsudo, Take-aki,Watanabe, Yoshihisa
, p. 3011 - 3013 (1988)
(1-2;5-6-η-Cyclooctadiene)(1-6-η-cyclooctatriene)ruthenium catalyzes the silylation of olefins at 60-140 deg C to give allylsilanes with good selectivity in high yields.The reactions of trialkylsilane with 2-methyl-1-butene, 2-methyl-2-butene, and 3-methyl-1-butene give the same product, 3-methyl-1-trialkylsilyl-2-butene.Silylation of ethyl (E)-2-butenoate gives ethyl (E)-4-trialkylsilyl-2-butenoate in high yield.
COMPETITIVE TYPE II ELIMINATIONS IN ALIPHATIC IMIDES
Mazzocchi, Paul H.,Jameson, William,Nishiyama, Tomikiro,DeCamp, Ann
, p. 989 - 992 (1980)
Aliphatic imides have been shown to undergo type II eliminations across the imide moiety in addition to those on the C-alkyl chain and α cleavage reactions.
Ester synthesis using Candida rugosa lipase immobilized on magnetic nanoparticles
Dandavate, Vrushali,Keharia, Haresh,Madamwar, Datta
, p. 37 - 45 (2011)
Magnetic nanoparticles were synthesized by co-precipitation under hydrothermal conditions. The average diameter of the magnetic nanoparticles was found to be in the range of 15 ± 5 nm with an average surface area of 112.15 m2 g-1. Immobilization of lipase on magnetite nanoparticles was confirmed by FTIR, differential scanning calorimetry and thermal gravimetric analysis. The activation energy of the free enzyme was 1.9-fold higher than that of the immobilized lipase for hydrolytic reactions. Additionally, the lower KM and higher Vmax values of the immobilized enzyme for hydrolysis of 4-nitrophenyl palmitate indicated an increased efficiency of the immobilized lipase. The immobilized lipase exhibited higher esterification efficiency compared with free lipase for synthesis of ethyl isovalerate. It also exhibited fairly good reusability, with about 8.5% reduction in esterification efficiency for ethyl isovalerate synthesis over ten cycles of reuse.
Stability improvement of immobilized Candida antarctica lipase B in an organic medium under microwave radiation
Rejasse, Barbara,Lamare, Sylvain,Legoy, Marie-Dominique,Besson, Thierry
, p. 1086 - 1089 (2004)
The influence of microwave heating on the stability of immobilized Candida antarctica lipase B was studied at 100°C in an organic medium. The microwave radiation was carried out before enzymatic reaction (storage conditions) or during the enzymatic catalysis (use conditions). In both cases, enzymatic stability was higher under microwave heating than under conventional thermal heating, in strictly identical operating conditions. Furthermore, the gain of enzymatic stability under microwave heating appears to be higher in a more polar solvent, which interacts strongly with the microwave field. Our results suggest that microwave radiation has an effect, not related to temperature, on the process of enzymatic inactivation.
-
Kutz,Adkins
, ()
-
Design, Synthesis, and Study of the Insecticidal Activity of Novel Steroidal 1,3,4-Oxadiazoles
Bai, Hangyu,Jiang, Weiqi,Li, Qi,Li, Tian,Ma, Shichuang,Shi, Baojun,Wu, Wenjun
, p. 11572 - 11581 (2021/10/12)
A series of novel steroidal derivatives with a substituted 1,3,4-oxadiazole structure was designed and synthesized, and the target compounds were evaluated for their insecticidal activity against five aphid species. Most of the tested compounds exhibited potent insecticidal activity against Eriosoma lanigerum (Hausmann), Myzus persicae, and Aphis citricola. Compounds 20g and 24g displayed the highest activity against E. lanigerum, showing LC50 values of 27.6 and 30.4 μg/mL, respectively. Ultrastructural changes in the midgut cells of E. lanigerum were detected by transmission electron microscopy, indicating that these steroidal oxazole derivatives might exert their insecticidal activity by destroying the mitochondria and nuclear membranes in insect midgut cells. Furthermore, a field trial showed that compound 20g exhibited effects similar to those of the positive controls chlorpyrifos and thiamethoxam against E. lanigerum, reaching a control rate of 89.5% at a dose of 200 μg/mL after 21 days. We also investigated the hydrolysis and metabolism of the target compounds in E. lanigerum by assaying the activities of three insecticide-detoxifying enzymes. Compound 20g at 50 μg/mL exhibited inhibitory action on carboxylesterase similar to the known inhibitor triphenyl phosphate. The above results demonstrate the potential of these steroidal oxazole derivatives to be developed as novel pesticides.
Cavity-promotion by pillar[5]arenes expedites organic photoredox-catalysed reductive dehalogenations
Esser, Birgit,Schmidt, Maximilian
supporting information, p. 9582 - 9585 (2021/09/28)
The efficiency of the photo-induced electron transfer in photoredox catalysis is limited by the diffusional collision of the excited catalyst and the substrate. We herein present cavity-bound photoredox catalysts, which preassociate the substrates, leading to significantly shortened reaction times. A pillar[5]arene serves as the cavity and phenothiazine as a catalyst in the reductive dehalogenation of aliphatic bromides as a proof of concept reaction.
Ball-Milling-Enabled Reactivity of Manganese Metal**
Bolt, Robert R. A.,Browne, Duncan L.,Howard, Joseph L.,Khan, Adam,Magri, Giuseppina,Morrill, Louis C.,Nicholson, William I.,Richards, Emma,Seastram, Alex C.
supporting information, p. 23128 - 23133 (2021/09/20)
Efforts to generate organomanganese reagents under ball-milling conditions have led to the serendipitous discovery that manganese metal can mediate the reductive dimerization of arylidene malonates. The newly uncovered process has been optimized and its mechanism explored using CV measurements, radical trapping experiments, EPR spectroscopy, and solution control reactions. This unique reactivity can also be translated to solution whereupon pre-milling of the manganese is required.
Chromium-Catalyzed Production of Diols From Olefins
-
Paragraph 0111, (2021/03/19)
Processes for converting an olefin reactant into a diol compound are disclosed, and these processes include the steps of contacting the olefin reactant and a supported chromium catalyst comprising chromium in a hexavalent oxidation state to reduce at least a portion of the supported chromium catalyst to form a reduced chromium catalyst, and hydrolyzing the reduced chromium catalyst to form a reaction product comprising the diol compound. While being contacted, the olefin reactant and the supported chromium catalyst can be irradiated with a light beam at a wavelength in the UV-visible spectrum. Optionally, these processes can further comprise a step of calcining at least a portion of the reduced chromium catalyst to regenerate the supported chromium catalyst.