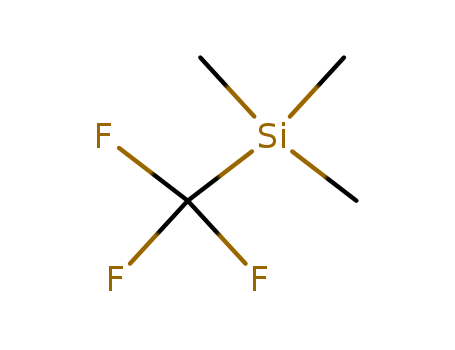
(Trifluoromethyl)trimethylsilane literature
TETRAKIS(DIMETHYLAMINO)ETHYLENE/TRIFLUOROIODOMETHANE, A SPECIFIC NOVEL TETRAFLUOROMETHYLATING AGENT
Pawelke, G.
, p. 429 - 434 (1989)
At low temperatures tetrakis(dimethylamino)ethylene (I) and CF3I form a charge transfer complex, which can act as a nucleophilic trifluoromethylating agent in polar solvents according to eqn. (1): The applicability of eqn.(1) to various silicon and boron halides R-X was tested and the following trifluoromethyl-silicon and boron derivatives were obtained in resonable yields: Me3SiCF3 (II), Me2Si(CF3)2 (III), (F3C)3BNHEt2 (IV).
An efficient inexpensive electrochemical preparation of Ruppert's reagent
Aymard, Frederic,Nedelec, Jean-Yves,Perichon, Jacques
, p. 8623 - 8624 (1994)
The electrochemical reduction of CF3Br in N,N-dimethylformamide (DMF) in the presence of Me3SiCl and a sacrificial aluminum anode provides Me3SiCF3 in ca 90% faradaic yields.
SYNTHESIS AND PROPERTIES OF (TRIFLUOROMETHYL)TRICHLOROSILANE, A VERSATILE PRECURSORFOR CF3Si COMPOUNDS
Beckers, H.,Buerger, H.,Bursch, P.,Ruppert, I.
, p. 41 - 50 (1986)
(Trifluoromethyl)trichlorosilane (I) has been prepared for the first time by the reaction of CF3SiH(NMe2)2 (III) with HCl in dibutyl ether and, as a solution in CH2Cl2, by nucleophilic trifluoromethylation of SiCl4 with CF3Br/P(NEt2)3.From this solution, I mey be isolated by HCl cleavage of its insoluble bis(pyridine) adduct (II).The aminosilane III was obtained from the reaction of HSi(Cl)(NMe2)2 with CF3Br/P(NEt2)3.Selective substitution of I without attack on the CF3Si moiety was achieved in high yields with SbF3 (-> CF3SiF3), Ag(OCN) (-> CF3Si(NCO)3 (IV)), MeOH (-> CF3Si(OMe)3 (V)), HNMe2 (CF3Si(NMe2)3 (VI)), LiAlH4 (-> CF3SiH3), MeMgBr (-> CF3SiMe3) and LiPh (-> CF3SiPh3 (VII)).With 2,2'-bipyridyl a 1/1complex (VIII) was formed.The novel compounds I to VIII have been characterized by their IR, NMR and mass spectra, and for I a vibrational analysis including a normal coordinate treatment has been performed.
Preparation method of trifluoromethyltrimethylsilane
-
Paragraph 0040-0063, (2021/07/14)
The invention provides a preparation method of trifluoromethyltrimethylsilane. The preparation method comprises the following steps: (1) synthesizing a Grignard reagent by taking magnesium metal and trifluorohalomethane as raw materials; and (2) enabling the Grignard reagent to react with trimethyl halogenosilane, so as to prepare the trifluoromethyl trimethyl silane. The preparation method of trifluoromethyltrimethylsilane provided by the invention has the advantages of high yield, simple process, low cost and the like.
Generation and Applications of the Hydroxide Trihydrate Anion, [OH(OH2)3]?, Stabilized by a Weakly Coordinating Cation
Weitkamp, Robin F.,Neumann, Beate,Stammler, Hans-Georg,Hoge, Berthold
supporting information, p. 14633 - 14638 (2019/11/05)
The reaction of a strongly basic phosphazene (Schwesinger base) with water afforded the corresponding metastable hydroxide trihydrate [OH(OH2)3]? salt. This is the first hydroxide solvate that is not in contact with a cation and furthermore one of rare known water-stabilized hydroxide anions. Thermolysis in vacuum results in the decomposition of the hydroxide salt and quantitative liberation of the free phosphazene base. This approach was used to synthesize the Schwesinger base from its hydrochloride salt after anion exchange in excellent yields of over 97 %. This deprotonation method can also be used for the phosphazene-base-catalyzed preparation of the Ruppert–Prakash reagent Me3SiCF3 using fluoroform (HCF3) as the trifluoromethyl building block and sodium hydroxide as the formal deprotonation agent.
Borazine-CF3? Adducts for Rapid, Room Temperature, and Broad Scope Trifluoromethylation
Geri, Jacob B.,Wade Wolfe, Michael M.,Szymczak, Nathaniel K.
supporting information, p. 1381 - 1385 (2018/01/15)
A fluoroform-derived borazine CF3? transfer reagent is used to effect rapid nucleophilic reactions in the absence of additives, within minutes at 25 °C. Inorganic electrophiles spanning seven groups of the periodic table can be trifluoromethylated in high yield, including transition metals used for catalytic trifluoromethylation. Organic electrophiles included (hetero)arenes, enabling C?H and C?X trifluoromethylation reactions. Mechanistic analysis supports a dissociative mechanism for CF3? transfer, and cation modification afforded a reagent with enhanced stability.
COMPLEXES FOR NUCLEOPHILIC, RADICAL, AND ELECTROPHILIC POLYFLUOROALKYLATION
-
Paragraph 00157; 00159; 00160, (2018/04/11)
Disclosed herein are borazine complexes and use of the same in perfluoroalkylation reactions.