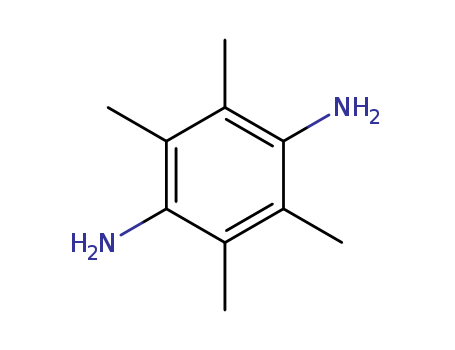
2,3,5,6-TETRAMETHYL-1,4-PHENYLENEDIAMINE literature
Activated sterically strained C=N bond in N-Arylsulfonyl-p-quinonemono-and diimines: IX. Synthesis and reactions of N-Tosyl-2,3,5,6-tetramethyl-1,4-benzoquinonimine
Avdeenko,Yusina,Yagupol'skii
, p. 1124 - 1129 (2007/10/03)
N-Tosyl-2,3,5,6-tetramethyl-1,4-benzoquinonimine as the other N-arylsulfonyl-1,4-benzoquinonimines with an activated C=N bond reacts along 1,2-addition path with alcohols and sodium azide and along 1,2-addition-elimination path with aromatic amines. The higher activity of the C=N bond in the N-arylsulfonyl-1,4-benzoquinonimines is not due to the electronic character of the substituent attached to the ring (Cl, CH3) but to steric influence resulting in increase in the bond angle C=N-S.
Triazinyl reactive dyestuffs in which triazinyl group is further substituted with a beta-chloroethylsulfonyl- or vinylsulfonylbutyrylamino moiety
-
, (2008/06/13)
Reactive dyes of the formula STR1 in which D is the radical of an organic dye of the monoazo, polyazo, metal complex azo, anthraquinone, phthalocyanine, formazan, azomethine, dioxazine, phenazine, stilbene, triphenylmethane, xanthene, thioxanthrone, nitroaryl, naphthoquinone, pyrenequinone or perylenetetracarbimide series, R is hydrogen or substituted or unsubstituted C1-4 -alkyl, X is a substituent which is detachable as an anion, B is a radical of the formula STR2 R1 and R2, independently of each other, are hydrogen or substituted or unsubstituted C1-4 -alkyl or phenyl, A is a substituted or unsubstituted aliphatic or aromatic bridge member, Y is a --CO--Z or --SO2 --Z radical, Z is an aliphatic, aromatic or heterocyclic reactive radical, and n is 1 or 2, are suitable for dyeing or printing cellulose-containing and nitrogen-containing materials and in high dyeing yield produce dyeings and prints having good fastness properties.
REDUCTION OF AROMATIC NITRO COMPOUNDS WITH SODIUM TELLURIDE
Suzuki, Hitomi,Manabe, Hajime,Inouye, Masahiko
, p. 1671 - 1674 (2007/10/02)
Sodium telluride, prepared by heating tellurium with Rongalite in aqueous sodium hydroxide, easily reduces aromatic nitro compounds to the corresponding amines in good yields.The reduction can be carried out using a catalytic amount of tellurium, since sodium telluride is readily regenerated in the presence of excess Rongalite.